Regulation of Survival Networks in Senescent Cells: from Mechanisms to Interventions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes
Sound Decisions: An Undergraduate Bioethics Journal Volume 2 Issue 1 Article 4 2016-12-15 Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes Eric Ralph University of Puget Sound Follow this and additional works at: https://soundideas.pugetsound.edu/sounddecisions Part of the Bioethics and Medical Ethics Commons Recommended Citation Ralph, Eric (2016) "Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes," Sound Decisions: An Undergraduate Bioethics Journal: Vol. 2 : Iss. 1 , Article 4. Available at: https://soundideas.pugetsound.edu/sounddecisions/vol2/iss1/4 This Article is brought to you for free and open access by the Student Publications at Sound Ideas. It has been accepted for inclusion in Sound Decisions: An Undergraduate Bioethics Journal by an authorized editor of Sound Ideas. For more information, please contact [email protected]. Ralph: Longevity Extension Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes Eric Ralph Introduction In the last several decades, a significant amount of progress has been made in pursuits to better understand the process of aging and subsequently gain some level of control over it. Current theories of aging are admittedly lacking, but this has not prevented biogerontologists from drastically increasing the longevity of yeast, drosophilae, worms, and mice (Vaiserman, Moskalev, & Pasyukova 2015; Tosato, Zamboni et al. 2007; Riera & Dillin 2015). Wide-ranging successes with gene therapy and increased comprehension of the genetic components of aging have also recently culminated in numerous successes in extending the longevity of animals and the first human trial of a gene therapy to extend life through telomerase manipulation is already underway, albeit on a small scale (Mendell et al. -

Worms Under Stress: Unravelling Genetic Complex Traits Through Perturbation
Worms under stress: unravelling genetic complex traits through perturbation Miriam Rodriguez Sanchez Thesis committee Promotor Prof. Dr Jaap Bakker Professor of Nematology Wageningen University Co-promotor Dr Jan E. Kammenga Associate professor, Laboratory of Nematology Wageningen University Other members Prof. Dr Bas J. Zwaan, Wageningen University Prof. Dr Hendrik.C. Korswagen, Hubrecht Institute, Utrecht Prof. Dr Ellen Nollen, European Research Institute for the Biology of Ageing (ERIBA), Groningen Dr Gino B. Poulin, University of Manchester, United Kingdom This research was conducted under the auspices of the Graduate School of Production Ecology and Resource Conservation (PE&RC). Worms under stress: unravelling genetic complex traits through perturbation Miriam Rodriguez Sanchez Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 14 March 2014 at 11 a.m. in the Aula. Miriam Rodriguez Sanchez Worms under stress: unravelling genetic complex traits through perturbation 130 pages PhD thesis, Wageningen University, Wageningen, NL (2014) With references, with summaries in Dutch and English ISBN: 978-94-6173-851-6 A mi padre CONTENTS Contents Chapter 1 General Introduction ........................................................................... 3 Chapter 2 C. elegans stress response and its relevance to complex human disease and aging ................................................. 15 Chapter 3 Uncovering genotype specific variation of Wnt signaling in C. elegans .................................................................... 33 Chapter 4 Genetic variation for stress-response hormesis in C. elegans life span ......................................................................... 55 Chapter 5 Molecular confirmation of trans-regulatory eQTL in C. -

University Medical Center of the University of Groningen Research Portfolio
University Medical Center of the University of Groningen Research portfolio Research Institutes and Research Programmes UMCG’s multidisciplinary Research Programmes are organised in five Research Institutes. Each Institute covers a specific part of the UMCG research area. By establishing numerous international research projects and strategic alliances over the past, research within the Institutes bridged national boundaries. Especially for the UMCG focus "Active and Healty Ageing" international collaboration is required and prepares the UMCG to contribute to this global challenge. For every UMCG Research Programme the relevance for Healthy Ageing is defined. Regarding the training and education of future scientists (MSc and PhD) the five Research Institutes collaborate in the Graduate School of Medical Sciences assuring the incorporation of state of the art scientific know-how. 1. GUIDE Institute: Chronic Diseases and Drug Exploration 2. BCN-BRAIN Institute: Behavioural and Cognitive Neurosciences 3. SHARE Institute: Health Research and Epidemiology 4. W.J.Kolff Institute: Biomaterials 5. CRCG Institute: Fundamental, Clinical and Translational Cancer Research Some of the Research Programmes are Platform Programmes, embedded in and supporting all five Research Institutes. Contents: Research programmes GUIDE 02-26 Research programmes BCN-BRAIN 27-35 Research programmes SHARE 36-46 Research programmes CRCG 47-55 Research programmes W.J. Kolff 56-65 Platform programme Center Medical Imaging 66-68 1 RESEARCH INSTITUTE GUIDE: Chronic Diseases and Drug Exploration 1. Biopharmaceuticals, Discovery, Design and Delivery (GUIDE-BDDD) Programme leaders: prof. dr. H.W. Frijlink, prof. dr. K. Poelstra Mission The BDDD Division explores innovative approaches oriented towards the early phase of drug development up to the use of these approaches in practice. -

Tuesday 24Th
Matchmaking event University of Groningen/UMCG and University of Chile 24-25 September, 2019 Time slot Tuesday 24th Location 09:00-10:30 Health Kick-off ERIBA Seminar Room 10:30-11:00 Break ERIBA Pantry 11:00-13:00 Parallel session Time Neuroscience & Biology of ageing (ERIBA seminar room) Time Oncology & Drug Delivery (Room 16) 11:00-11:25 Felipe Court: 11:00-11:20 Andrew Quest: Necroaxoptosis: a novel axonal degenerative mechanism involved in pathologies of the ageing nervous system From tumor suppressor to metastasis promoter – Caveolin-1, a Jack-of-all trades in cancer 11:25-11:40 Marco Demaria: 11:20-11:40 Frank Kruyt: Role of cellular senescence in health and disease Brain tumors: glioblastoma stem cell models and identification of new therapeutic targets 11:40-12:02 Miguel Concha: 11:40-12:00 Marcelo Kogan: Nothobranchious furzeru as a model of aging and neurodegeneration Nanoplatforms for drug delivery, theraphy and diagnostic of chronic diseases 12:05-12:20 Harrie Kampinga: 12:00-12:20 Paul de Vos: Protein homeostasis and age-related protein aggregation diseases Carbohydrates and microbiota in health 12:20-12:45 Christian González-Billault: 12:20-12:40 Inge Zuhorn: Understanding neuronal aging using cell culture models Drug Delivery across the Blood-Brain Barrier 12:45-13:00 Amalia Dolga: 12:40-13:00 Wijnand Helfrich: Targeting mitochondria in human neurodegenerative disease model systems Novel Targeted approaches in Cancer Immunotherapy 13-14:30 Lunch ERIBA Pantry 15:00-17:30 Parallel session Time Neuroscience & Biology of ageing -

Annual Report V2.Indd 1 16-07-2020 20:59 Welcome to Our Annual Report 2019
European Research Institute for the Biology of Ageing Annual Report 2019 ERIBA | Cover .indd Alle pagina's 30-06-2020 13:49 Annual Report 2019 2019 ERIBA | Annual Report V2.indd 1 16-07-2020 20:59 Welcome to our Annual Report 2019 Coordination: Gerald de Haan and Megha Upadhyay Secretarial Support: Sylvia Hoks, Annet Vos-Hassing & Alida de Haan ژيƺɀǣǕȇƏȇƳXǼǼɖɀɎȸƏɎǣȒȇɀ) Stefan Heinrich ژيȸǣȇɎǣȇǕ¨ Ridderprint BV | Anand Baldew Copies: 100 ERIBA | Annual Report V2.indd 2 16-07-2020 20:59 Annual Report Table of contents 1. Foreword by the Director 4 2. Ageing Research at ERIBA 6 3. 2019: Highlights 10 4.Facts and Figures 20 אא ³ƬǣƺȇɎǣˡƬ¨ɖƫǼǣƬƏɎǣȒȇɀٮ -Funding/Grants 32 -Invited Speakers 35 אג ƺȒȵǼƺ¨ٮ 5.Facilities 46 6.Education 50 7.Outreach & Dissemination 54 זד ³ƬǣƺȇɎǣˡƬƳɮǣɀȒȸɵ ȒƏȸƳِז 9.Sponsors 59 ERIBA | Annual Report V2.indd 3 16-07-2020 20:59 Foreword by the Director 2019 in review It is a great pleasure to present to you the 2019 Annual Report of the European Research Institute for the Biology of Ageing. This report provides you with an overview of all our activities and achievements, in science, education, business development and outreach. We value all these domains equally, and are proud to share with you all that has been accomplished in 2019. I write these words in the midst of the Covid-19 pandemic. This pandemic may very well have a major effect on global research and education for quite some time to come. One aspect, highly relevant to what we do in ERIBA, relates to how the virus differentially affects individuals in society. -

Shared Ageing Research Models (Sharm): a New Facility to Support Ageing Research
Biogerontology (2013) 14:789–794 DOI 10.1007/s10522-013-9457-0 METHOD Shared Ageing Research Models (ShARM): a new facility to support ageing research Adele L. Duran • Paul Potter • Sara Wells • Tom Kirkwood • Thomas von Zglinicki • Anne McArdle • Cheryl Scudamore • Qing-Jun Meng • Gerald de Haan • Anne Corcoran • Ilaria Bellantuono Received: 5 July 2013 / Accepted: 16 August 2013 / Published online: 2 October 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract In order to manage the rise in life expec- Wellcome Trust, open to all investigators. It collects, tancy and the concomitant increased occurrence of stores and distributes flash frozen tissues from aged age-related diseases, research into ageing has become murine models through its biorepository and provides a strategic priority. Mouse models are commonly a database of live ageing mouse colonies available in utilised as they share high homology with humans and the UK and abroad. It also has an online environment show many similar signs and diseases of ageing. (MICEspace) for collation and analysis of data from However, the time and cost needed to rear aged communal models and discussion boards on subjects cohorts can limit research opportunities. Sharing of such as the welfare of ageing animals and common resources can provide an ethically and economically endpoints for intervention studies. Since launching in superior framework to overcome some of these issues July 2012, thanks to the generosity of researchers in but requires dedicated infrastructure. Shared Ageing UK and Europe, ShARM has collected more than Research Models (ShARM) (www.ShARMUK.org) 2,500 tissues and has in excess of 2,000 mice regis- is a new, not-for-profit organisation funded by tered in live ageing colonies. -
Viewer Comments
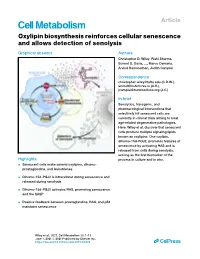
Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Graphical abstract Authors Christopher D. Wiley, Rishi Sharma, Sonnet S. Davis, ..., Marco Demaria, Arvind Ramanathan, Judith Campisi Correspondence [email protected] (C.D.W.), [email protected] (A.R.), [email protected] (J.C.) In brief Senolytics, transgenic, and pharmacological interventions that selectively kill senescent cells are currently in clinical trials aiming to treat age-related degenerative pathologies. Here, Wiley et al. discover that senescent cells produce multiple signaling lipids known as oxylipins. One oxylipin, dihomo-15d-PGJ2, promotes features of senescence by activating RAS and is released from cells during senolysis, serving as the first biomarker of the Highlights process in culture and in vivo. d Senescent cells make several oxylipins, dihomo- prostaglandins, and leukotrienes d Dihomo-15d-PGJ2 is intracellular during senescence and released during senolysis d Dihomo-15d-PGJ2 activates RAS, promoting senescence and the SASP d Positive feedback between prostaglandins, RAS, and p53 maintains senescence Wiley et al., 2021, Cell Metabolism 33, 1–13 June 1, 2021 ª 2021 Published by Elsevier Inc. https://doi.org/10.1016/j.cmet.2021.03.008 ll Please cite this article in press as: Wiley et al., Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis, Cell Metabolism (2021), https://doi.org/10.1016/j.cmet.2021.03.008 ll Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Christopher D. Wiley,1,2,* Rishi Sharma,1 Sonnet S. Davis,1 Jose Alberto Lopez-Dominguez,1 Kylie P. Mitchell,1 Samantha Wiley,1 Fatouma Alimirah,1 Dong Eun Kim,1 Therese Payne,1 Andrew Rosko,1 Eliezer Aimontche,1 Sharvari M. -

Age-Dependent Deterioration of Nuclear Pore Assembly in Mitotic
RESEARCH ARTICLE Age-dependent deterioration of nuclear pore assembly in mitotic cells decreases transport dynamics Irina L Rempel1, Matthew M Crane2, David J Thaller3, Ankur Mishra4, Daniel PM Jansen1, Georges Janssens1, Petra Popken1, Arman Aks¸it1, Matt Kaeberlein2, Erik van der Giessen4, Anton Steen1, Patrick R Onck4, C Patrick Lusk3, Liesbeth M Veenhoff1* 1European Research Institute for the Biology of Ageing (ERIBA), University of Groningen, University Medical Center Groningen, Groningen, Netherlands; 2Department of Pathology, University of Washington, Seattle, United States; 3Department of Cell Biology, Yale School of Medicine, New Haven, United States; 4Zernike Institute for Advanced Materials, University of Groningen, Groningen, Netherlands Abstract Nuclear transport is facilitated by the Nuclear Pore Complex (NPC) and is essential for life in eukaryotes. The NPC is a long-lived and exceptionally large structure. We asked whether NPC quality control is compromised in aging mitotic cells. Our images of single yeast cells during aging, show that the abundance of several NPC components and NPC assembly factors decreases. Additionally, the single-cell life histories reveal that cells that better maintain those components are longer lived. The presence of herniations at the nuclear envelope of aged cells suggests that misassembled NPCs are accumulated in aged cells. Aged cells show decreased dynamics of transcription factor shuttling and increased nuclear compartmentalization. These functional changes are likely caused by the presence -

Book of Abstracts 2021
BOOK OF ABSTRACTS Preface Organisation Research in Groningen Congress Abstracts Plenary Abstracts Oral Abstracts Poster Postscript 2 Table of Contents Preface � � � � � � � � � � � � � � � � � � � � � � � � � � � 5 Cell Biology � � � � � � � � � � � � � � � � � � � � � � � 99 Tessa de Bruin � � � � � � � � � � � � � � � � � � � � � � 6 Endocrinology & Diabetes � � � � � � � � � � � � � �106 Prof� Marian Joëls MD PhD � � � � � � � � � � � � � � � 7 Pediatrics, Obstetrics & Reproductive health � �110 Organisation � � � � � � � � � � � � � � � � � � � � � � � 8 Neurology & Neurosurgery � � � � � � � � � � � � �115 Executive Board � � � � � � � � � � � � � � � � � � � � � 9 Cardiology & Vascular medicine � � � � � � � � � �122 Advisory Board � � � � � � � � � � � � � � � � � � � � � 10 Oncology I � � � � � � � � � � � � � � � � � � � � � � � �128 President, Secretary, Treasurer � � � � � � � � � � � 11 Pulmonology � � � � � � � � � � � � � � � � � � � � � �134 Scientific Programme � � � � � � � � � � � � � � � � � 12 Oral Sessions II � � � � � � � � � � � � � � � � � � � � 139 Sponsors & Fundraising � � � � � � � � � � � � � � � 13 Public health II � � � � � � � � � � � � � � � � � � � � �140 International Contacts � � � � � � � � � � � � � � � � 14 Oncology II � � � � � � � � � � � � � � � � � � � � � � �146 Hosting & Logistics � � � � � � � � � � � � � � � � � � 15 Epidemiology � � � � � � � � � � � � � � � � � � � � � �153 Public Relations � � � � � � � � � � � � � � � � � � � � 16 Pharmacology � � � � � � � � � � � � � � � � � � � � �160 -

European Research Institute for the Biology of Ageing
European Research Institute for the Biology of Ageing To better understand what causes ageing Foreword A better understanding of the cellular processes that cause ageing is important from a scientific as well as a “healthy ageing” perspective. This is the research area of the European Research Institute for the Biology of Ageing (ERIBA). This brochure explains why such research is important and what it is that we do. Research on the biology of ageing has been carried out for decades by numerous scientists all over the world. Yet our knowledge of the cellular processes that have been implicated is still far from complete. Fragmentation and compartmentalisation of the very wide research area is partly to blame. However, the insights that do exist also need to be better integrated. As a new institute, ERIBA aims to play a major role in the acquisition of new knowledge as well as in the integration of existing knowledge in all areas related to the biology of ageing. In 2007 the first plans were made at the University Medical Centre in Groningen (UMCG) for a new research Institute that could support, via fundamental research, the strategic choice for ‘Healthy Ageing’. Collaboration was sought betterTo understand what causes ageing. with the University of Groningen and other local, national and European ||| 3 partners. In 2009 this resulted in a concrete business plan. In 2010 the construction plans were drawn up and ERIBA was born in 2013. We started life in a modern building fully equipped to suit our vision that multidisciplinary work is a key ingredient to achieve breakthroughs in bio medical research. -

Download Magazine
Do You Still Need Aspirin? LifeExtension.com Stay Healthy, Live Better August 2019 Faster, Longer, More Restful SLEEP Enhance Male Sexual Health PQQ Refreshes Brain Energy Maintain Healthy Coagulation Balance Carotenoids to Protect Eye and Brain Function Omega-3 Intake Associated with Lower Mortality Risk REJUVENATE YOUR SKIN FROM WITHIN Restore Collagen AND Hyaluronic Acid WITH DELIGHTFUL GUMMIES Oral ingestion of collagen peptides and hyaluronic acid boosts these rejuvenating factors in normal, aging skin. Clinical results reveal improved skin elasticity, increased moisture, and a % reduction in the depth of eye wrinkles. The new Gummy Science™ Youthful Collagen formula provides clinically studied* doses with daily intake of tasty chewable gummies. For full product description and to order Gummy Science™ Youthful Collagen, call --- or visit www.LifeExtension.com Item # • gummies gummiess jar $. * Skin Pharmacol Physiol. ;():-. * Skin Pharmacol Physiol. ;():-. VERISOL® and Bioactive Collagen Peptides® are registered trademarks of GELITA AG. jars $ each These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. Table of Contents Volume Twenty Five / Number Eight • August 2019 REPORTS 38 ENHANCE MALE SEXUAL HEALTH A ginger-like root was shown to improve sexual health in 61.5% of male participants in a published study. 46 PQQ REVITALIZES CELLULAR ENERGY A nutrient called PQQ helps grow new mitochondria in aging cells. The result is more energy in human studies and increased lifespan (in animal studies). 57 LUTEIN AND ZEAXANTHIN IMPROVE COGNITION Lutein and zeaxanthin are plant carotenoids known for protecting the eyes. New studies reveal they also increase brain processing speed, visual memory, cognitive 28 ON THE COVER flexibility, and brain blood flow. -

El Transhumanisme Sota La Lupa Francesc Torralba, Coordinador Conferències Curs 2017-2018
El Transhumanisme sota la lupa Francesc Torralba, coordinador Conferències curs 2017-2018 T E H E M O C R L U B O F BARCELONA Francesc Torralba Roselló és filòsof i teòleg. Neix a Barcelona el 15 de maig de 1967. Casat i pare de 5 fills. Doctor en Filosofia per la Universitat de Barcelona (1992). Doctor en Teologia per la Facultat de Teologia de Catalunya (1997). Doctor en Pedagogia per la Universitat Ramon Llull (2018). Amplia estudis a les Universitats de Copenhaguen i Berlín. En l’actualitat és catedràtic acreditat a la Universitat Ramon Llull, i imparteix cursos i seminaris en altres universitats d’Espanya i d’Amèrica. Alterna la seva activitat docent amb l’ofici d’escriure, i divulgar el seu pensament. Francesc Torralba és un autor prolífic, amb més de 1.500 articles i 100 llibres publicats dels quals destaquem: "El sentit de la vida" (2008), Biblioteca Francesc Torralba dedicada a valors amb títols com "La Tendresa", "La Paciència" o "L'Amistat", (2008), "Inteligencia Espiritual" (2010), "Jesucrist 2.0" (2011), "El valor de tenir valors" (2012), "Un mar d'e- mocions" (2013), "Córrer per pensar i sentir" (2015), "Saber dir no" (2016) i "La vida secreta de la pregària" (2017). Part de la seva obra ha estat traduïda al castellà, l'a- lemany, el francès, l'italià, el portuguès, el romanès i l'anglès. ament. EL TRANSHUMANISME SOTA LA LUPA Conferències curs 2017-2018 Amb la col.laboració de: Disseny i impressió: Vanguard Gràfic, SA ÍNDEX Introducción, de Jaime Lanaspa . 5 ¿De què parlem quan parlem de transhumanisme?, de Francesc Torralba .