Both ERK and Wnt/Β-Catenin Pathways Are Involved in Wnt3a-Induced Proliferation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WNT4 and WNT3A Activate Cell Autonomous Wnt Signaling Independent of Secretion
bioRxiv preprint doi: https://doi.org/10.1101/333906; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Running Title: Secretion-independent Wnt signaling Research article WNT4 and WNT3A activate cell autonomous Wnt signaling independent of secretion Deviyani M. Rao1, Rebecca L. Ferguson1, Tomomi M. Yamamoto2, Benjamin G. Bitler2, Matthew J. Sikora1 Affiliation: 1Dept. of Pathology, 2Dept. of Obstetrics and Gynecology, University of Colorado Anschutz Medical Campus Corresponding author: Matthew J. Sikora, PhD.; Mail Stop 8104, Research Complex 1 South, Room 5117, 12801 E. 17th Ave.; Aurora, CO 80045. Tel: (303)724-4301; Fax: (303)724-3712; email: [email protected]. Twitter: @mjsikora Funding This work was supported by R00 CA193734 (MJS) and R00 CA194318 (BGB) from the National Institutes of Health, and by a grant from the Cancer League of Colorado, Inc (MJS). Authors' contributions DMR and MJS conceived of the project and experiments. DMR, RLF, and MJS designed and performed experiments. RLF, DMR, and TMY developed models for the project. DMR, RLF, BGB, and MJS contributed to data analysis and interpretation. DMR wrote the draft manuscript; all authors read and revised the manuscript, and have read and approved of this version of the manuscript. bioRxiv preprint doi: https://doi.org/10.1101/333906; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response
International Journal of Molecular Sciences Review Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response Casey D. Stefanski 1,2 and Jenifer R. Prosperi 1,2,3,* 1 Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46617, USA; [email protected] 2 Mike and Josie Harper Cancer Research Institute, South Bend, IN 46617, USA 3 Department of Biochemistry and Molecular Biology, Indiana University School of Medicine-South Bend, South Bend, IN 46617, USA * Correspondence: [email protected]; Tel.: +1-574-631-4002 Received: 30 September 2020; Accepted: 20 October 2020; Published: 22 October 2020 Abstract: Resistance to chemotherapy occurs through mechanisms within the epithelial tumor cells or through interactions with components of the tumor microenvironment (TME). Chemoresistance and the development of recurrent tumors are two of the leading factors of cancer-related deaths. The Adenomatous Polyposis Coli (APC) tumor suppressor is lost in many different cancers, including colorectal, breast, and prostate cancer, and its loss correlates with a decreased overall survival in cancer patients. While APC is commonly known for its role as a negative regulator of the WNT pathway, APC has numerous binding partners and functional roles. Through APC’s interactions with DNA repair proteins, DNA replication proteins, tubulin, and other components, recent evidence has shown that APC regulates the chemotherapy response in cancer cells. In this review article, we provide an overview of some of the cellular processes in which APC participates and how they impact chemoresistance through both epithelial- and TME-derived mechanisms. Keywords: adenomatous polyposis coli; chemoresistance; WNT signaling 1. -

Tsukushi Functions As a Wnt Signaling Inhibitor by Competing with Wnt2b for Binding to Transmembrane Protein Frizzled4
Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4 Kunimasa Ohtaa,b,1,2, Ayako Itoa,c,1, Sei Kuriyamaa,d,3, Giuseppe Lupoe,f, Mitsuko Kosakag,4, Shin-ichi Ohnumah, Shinichi Nakagawai, and Hideaki Tanakaa,c,d aDepartment of Developmental Neurobiology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto 860-8556, Japan; bPrecursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, Saitama 332-0012, Japan; cGlobal Center of Excellence, Kumamoto University, Kumamoto 860-8556, Japan; d21st Century Center of Excellence, Kumamoto University, Kumamoto 860-8556, Japan; eDepartment of Biology and Biotechnology “C. Darwin,” University of Rome “La Sapienza,” 00185 Rome, Italy; fIstituto Pasteur–Fondazione Cenci Bolognetti, 00185, Rome, Italy; gRIKEN Center for Developmental Biology, Kobe 650-0047, Japan; hInstitute of Ophthalmology, University College London, London EC1V 9EL, United Kingdom; and IRIKEN Advanced Science Institute, Nakagawa RNA Biology Laboratory, Saitama 351-0198, Japan Edited* by Lynn T. Landmesser, Case Western Reserve University, Cleveland, OH, and approved July 8, 2011 (received for review January 11, 2011) The Wnt signaling pathway is essential for the development of We previously described the isolation of Tsukushi (TSK) protein diverse tissues during embryogenesis. Signal transduction is acti- isoforms (13), soluble molecules belonging to the small leucine- vated by the binding of Wnt proteins to the type I receptor low- rich proteoglycan (SLRP) family (14), and showed that they work density lipoprotein receptor–related protein 5/6 and the seven-pass as extracellular modulators of pivotal signaling cascades during transmembrane protein Frizzled (Fzd), which contains a Wnt- early embryonic development in chicks and frogs (13, 15–17). -

Hippocampus Formation: an Intriguing Collaboration Henk Roelink
bb10g06.qxd 04/03/2000 01:10 Page R279 Dispatch R279 Hippocampus formation: An intriguing collaboration Henk Roelink Recent genetic studies have shown that the signalling temporal lobes are formed. In an adult rodent, the hip- factor Wnt3a is required for formation of the pocampus is consequently still found close to the dorsal hippocampus; the developmental consequences of Wnt midline where it is initially formed (Figure 1). signalling in the hippocampus are mediated by multiple HMG-box transcription factors, with LEF-1 being It is clear that Wnt family members are required for the required just for formation of the dentate gyrus. development of many embryonic structures, functions mediated by their effects on fundamental processes such Address: Department of Biological Structure and Center for Developmental Biology, University of Washington, Box 357420, as cell proliferation, differentiation, survival or mainte- Seattle, Washington 98117-7420, USA. nance. Obtaining a clear picture of how each Wnt acts is E-mail: [email protected] complicated by the relatively large size of the family, which has at least 18 members in amniotes [3]. Analyses of Current Biology 2000, 10:R279–R281 mice lacking one or several Wnt genes have revealed some 0960-9822/00/$ – see front matter remarkable phenotypes, often characterized by failure of © 2000 Elsevier Science Ltd. All rights reserved. induction of very specific anatomical structures. Wnt3A is no exception, and although several papers have been pub- Inductive interactions are fundamental to the formation of lished already on the more obvious defects of Wnt3a all brain structures. and signalling by molecules of the knockout mice, a recent paper from McMahon and col- Wingless/Wnt family is known to play a role in many of leagues [1] has reported a very interesting defect in the them. -

A Membrane-Associated Β-Catenin/Oct4 Complex Correlates
DEVELOPMENT AND STEM CELLS RESEARCH ARTICLE 1171 Development 140, 1171-1183 (2013) doi:10.1242/dev.085654 © 2013. Published by The Company of Biologists Ltd A membrane-associated β-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells Fernando Faunes1,*, Penelope Hayward1,*, Silvia Muñoz Descalzo1, Sujash S. Chatterjee2, Tina Balayo1, Jamie Trott1, Andrew Christoforou1,3, Anna Ferrer-Vaquer4, Anna-Katerina Hadjantonakis4, Ramanuj Dasgupta2 and Alfonso Martinez Arias1,‡ SUMMARY The maintenance of pluripotency in mouse embryonic stem cells (mESCs) relies on the activity of a transcriptional network that is fuelled by the activity of three transcription factors (Nanog, Oct4 and Sox2) and balanced by the repressive activity of Tcf3. Extracellular signals modulate the activity of the network and regulate the differentiation capacity of the cells. Wnt/β-catenin signaling has emerged as a significant potentiator of pluripotency: increases in the levels of β-catenin regulate the activity of Oct4 and Nanog, and enhance pluripotency. A recent report shows that β-catenin achieves some of these effects by modulating the activity of Tcf3, and that this effect does not require its transcriptional activation domain. Here, we show that during self-renewal there is negligible transcriptional activity of β-catenin and that this is due to its tight association with membranes, where we find it in a complex with Oct4 and E-cadherin. Differentiation triggers a burst of Wnt/β-catenin transcriptional activity that coincides with the disassembly of the complex. Our results establish that β-catenin, but not its transcriptional activity, is central to pluripotency acting through a β- catenin/Oct4 complex. -

Beta;-Catenin Signaling in Rhabdomyosarcoma
Laboratory Investigation (2013) 93, 1090–1099 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Characterization of Wnt/b-catenin signaling in rhabdomyosarcoma Srinivas R Annavarapu1,8,9, Samantha Cialfi2,9, Carlo Dominici2,3, George K Kokai4, Stefania Uccini5, Simona Ceccarelli6, Heather P McDowell2,3,7 and Timothy R Helliwell1 Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and accounts for about 5% of all malignant paediatric tumours. b-Catenin, a multifunctional nuclear transcription factor in the canonical Wnt signaling pathway, is active in myogenesis and embryonal somite patterning. Dysregulation of Wnt signaling facilitates tumour invasion and metastasis. This study characterizes Wnt/b-catenin signaling and functional activity in paediatric embryonal and alveolar RMS. Immunohistochemical assessment of paraffin-embedded tissues from 44 RMS showed b-catenin expression in 26 cases with cytoplasmic/membranous expression in 9/14 cases of alveolar RMS, and 15/30 cases of embryonal RMS, whereas nuclear expression was only seen in 2 cases of embryonal RMS. The potential functional significance of b-catenin expression was tested in four RMS cell lines, two derived from embryonal (RD and RD18) RMS and two from alveolar (Rh4 and Rh30) RMS. Western blot analysis demonstrated the expression of Wnt-associated proteins including b-catenin, glycogen synthase kinase-3b, disheveled, axin-1, naked, LRP-6 and cadherins in all cell lines. Cell fractionation and immunofluorescence studies of the cell lines (after stimulation by human recombinant Wnt3a) showed reduced phosphorylation of b-catenin, stabilization of the active cytosolic form and nuclear translocation of b-catenin. Reporter gene assay demonstrated a T-cell factor/lymphoid-enhancing factor-mediated transactivation in these cells. -

Wnt3a: Functions and Implications in Cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T
He et al. Chin J Cancer (2015) 34:50 DOI 10.1186/s40880-015-0052-4 REVIEW Open Access Wnt3a: functions and implications in cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T. Keller3, Chao‑Nan Qian4* and Jian Zhang1,2,3* Abstract Wnt3a, one of Wnt family members, plays key roles in regulating pleiotropic cellular functions, including self-renewal, proliferation, differentiation, and motility. Accumulating evidence has suggested that Wnt3a promotes or suppresses tumor progression via the canonical Wnt signaling pathway depending on cancer type. In addition, the roles of Wnt3a signaling can be inhibited by multiple proteins or chemicals. Herein, we summarize the latest findings on Wnt3a as an important therapeutic target in cancer. Keywords: Wnt3a, The Wnt signaling pathway, Cancer Background: the Wnt signaling pathway cell polarity pathway (Wnt-PCP pathway) and the Wnt- The Wnt gene was first identified in 1982 and is named calcium pathway (Wnt-Ca2+ pathway) [5]. According to the Int gene in mice [1]. A subsequent study reported the characteristics of its functions, the Wnt family can that the Int gene and wingless gene in Drosophila were also be divided into two categories: Wnt1 and Wnt5a. homologous genes [2]. Therefore, both genes were rec- The Wnt1 category includes Wnt1, Wnt2, Wnt2b, Wnt3, ognized as the Wnt gene [3]. The Wnt family comprises Wnt3a, Wnt7a, Wnt8, Wnt8b, and Wnt10a, which are 19 human proteins, including Wnt1, Wnt2, Wnt2b involved in the canonical signaling pathway, whereas (Wnt13), Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt4, Wnt5a, and Wnt11 belong to the Wnt5a category Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a (Wnt14), Wnt9b and activate the noncanonical signaling pathway. -

A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing
LETTER doi:10.1038/nature12631 A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing Maria Carolina Florian1, Kalpana J. Nattamai2, Karin Do¨rr1, Gina Marka1, Bettina U¨berle1, Virag Vas1, Christina Eckl3, Immanuel Andra¨4, Matthias Schiemann4, Robert A. J. Oostendorp3, Karin Scharffetter-Kochanek1, Hans Armin Kestler5, Yi Zheng2 & Hartmut Geiger1,2 Many organs with a high cell turnover (for example, skin, intestine LT-HSCs presented with a reduced level and primarily cytoplasmic loca- and blood) are composed of short-lived cells that require continuous lization of b-catenin (Fig. 1d, e and Supplementary Video 3). Reduced replenishment by somatic stem cells1,2. Ageing results in the inability levels of b-catenin upon ageing were specific to the LT-HSC compart- of these tissues to maintain homeostasis and it is believed that somatic ment, as more differentiated LKs (Lin2c-Kit1Sca-12 cells), LSKs stem-cell ageing is one underlying cause of tissue attrition with age (Lin2Sca-11c-Kit1 cells), lymphoid-primed multipotent progenitors or age-related diseases. Ageing of haematopoietic stem cells (HSCs) (LMPPs; Lin2c-Kit1Sca-12CD341Flk21 cells) and short-term (ST)- is associated with impaired haematopoiesis in the elderly3–6. Despite HSCs (Lin2c-Kit1Sca-12CD341Flk22 cells) (Extended Data Fig. 1h) a large amount of data describing the decline of HSC function on showed similar levels of b-catenin upon ageing (Fig. 1g and Extended ageing, the molecular mechanisms of this process remain largely Data Fig. 1i). Axin2 (an established direct downstream target of cano- unknown, which precludes rational approaches to attenuate stem- nical Wnt signalling9,15) transcript levels in aged LT-HSCs were mark- cell ageing. -
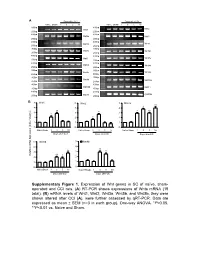
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1 -

Oncogenic Wnt3a
Journal of Cancer 2019, Vol. 10 5862 Ivyspring International Publisher Journal of Cancer 2019; 10(23): 5862-5873. doi: 10.7150/jca.31599 Research Paper Oncogenic Wnt3a: A Candidate Specific Marker and Novel Molecular Target for Hepatocellular Carcinoma Wenjie Zheng1*, Min Yao2*, Miao Fang1, Liuhong Pan1, Li Wang3, Junling Yang1, Zhizhen Dong4, Dengfu Yao1 1. Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China; 2. Department of Medical Immunology, Medical School of Nantong University, Nantong 226001, Jiangsu Province, China; 3. Department of Medical Informatics, Medical School of Nantong University, Nantong 226001, Jiangsu Province, China; 4. Department of Diagnostics, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China. * Equal contributors Corresponding author: Prof. Dengfu Yao, M.D. & Ph.D., Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, 20 West Temple Rd, Jiangsu 226001, China. Tel: +0086-513-85052523; Fax: +0086-513-85052254; E-mail: [email protected] © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2019.06.12; Accepted: 2019.08.20; Published: 2019.10.08 Abstract Background and aim: It is of the utmost importance for the specific diagnosis and effective therapy of hepatocellular carcinoma (HCC). Abnormality of oncogenic Wingless-type MMTV integration site family member 3a (Wnt3a) has been associated with progression of HCC. In this study, we aimed to evaluate Wnt3a as a novel biomarker and target for HCC. Methods: Circulating Wnt3a levels were quantitatively detected in a cohort of chronic liver diseases by an enzyme-linked immune-absorbent assay (ELISA). -

International Union of Basic and Clinical Pharmacology. LXXX. the Class Frizzled Receptors□S
Supplemental Material can be found at: /content/suppl/2010/11/30/62.4.632.DC1.html 0031-6997/10/6204-632–667$20.00 PHARMACOLOGICAL REVIEWS Vol. 62, No. 4 Copyright © 2010 by The American Society for Pharmacology and Experimental Therapeutics 2931/3636111 Pharmacol Rev 62:632–667, 2010 Printed in U.S.A. International Union of Basic and Clinical Pharmacology. LXXX. The Class Frizzled Receptors□S Gunnar Schulte Section of Receptor Biology and Signaling, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden Abstract............................................................................... 633 I. Introduction: the class Frizzled and recommended nomenclature............................ 633 II. Brief history of discovery and some phylogenetics ......................................... 634 III. Class Frizzled receptors ................................................................ 634 A. Frizzled and Smoothened structure ................................................... 635 1. The cysteine-rich domain ........................................................... 635 2. Transmembrane and intracellular domains........................................... 636 3. Motifs for post-translational modifications ........................................... 636 B. Class Frizzled receptors as PDZ ligands............................................... 637 C. Lipoglycoproteins as receptor ligands ................................................. 638 Downloaded from IV. Coreceptors........................................................................... -

Mycobacterium Tuberculosis of Wnt6 Is Expresse
Wnt6 Is Expressed in Granulomatous Lesions of Mycobacterium tuberculosis−Infected Mice and Is Involved in Macrophage Differentiation and Proliferation This information is current as of September 27, 2021. Kolja Schaale, Julius Brandenburg, Andreas Kispert, Michael Leitges, Stefan Ehlers and Norbert Reiling J Immunol 2013; 191:5182-5195; Prepublished online 11 October 2013; doi: 10.4049/jimmunol.1201819 Downloaded from http://www.jimmunol.org/content/191/10/5182 Supplementary http://www.jimmunol.org/content/suppl/2013/10/11/jimmunol.120181 http://www.jimmunol.org/ Material 9.DC1 References This article cites 60 articles, 23 of which you can access for free at: http://www.jimmunol.org/content/191/10/5182.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 27, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights