Farming Electrons: Galvanic Vs. Microbial Energy in Soil Batteries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Power Supply Using Earth Battery and Solar Pael
ITSI Transactions on Electrical and Electronics Engineering (ITSI-TEEE) _______________________________________________________________________________________________ Power Supply Using Earth Battery 1Harish D, 2T S Dhanalakshmi, 3Gireesh H R 1,2,3RRCE, Bangalore electronic devices. They can also be considered to Abstract: In view of robust and cost effective use of this natural power technology by unskilled village consumers replace high voltage low current charging power most suitable combinations of the commonly available supplies or ionization power supplies. Like earth metals were selected for further detailed characteristic batteries the sea batteries also may be considered for studies. Combinations of Magnesium anode and Coke similar applications. However, air batteries can be used cathode: Zinc anode and Graphite, cathode: Aluminum for bulk power production and grid system operation [3]. anode and Carbon cathode; Zinc anode and Copper In view of global energy crisis to be caused by natural cathodes gave 2.05, 1.40, 1.10 and 0.9 volts per cell. Typical end of oil and gas within next 50 to60 years time [9-11], rated power of a single Zn-Cu cell was measured to be few it has become very important to look for alternative tens of microamperes. Small power electronic devices such energy sources to hold back the human race from as calculators, electronic watches, baby toys and cell phones and white light LEDs were operated on site. The engagement to a great energy war [12-13]. Although, voltage level was found to increase linearly by connecting uranium [14] and coal [9] would continue to exist for multiple earth battery cells in series like commercial lead few centuries but they cannot replace oil and gas despite acid battery. -
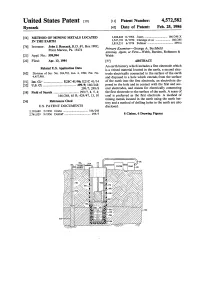
(1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb
United States Patent (19) (1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb. 25, 1986 54) METHOD OF MINING METALS LOCATED 3,288,648 11/1966 Jones ............................... 166/248 X N THE EARTH 3,547,192 12/1970 Claridge et al. .................... 166/248 3,819,231 6/1974 Fehliner ................................... 299/4 76 Inventor: John J. Ryeczek, R.D. #1, Box 190C, Primary Examiner-George A. Suchfield Point Marion, Pa. 15474 Attorney, Agent, or Firm-Webb, Burden, Robinson & 21 Appl. No.: 599,994 Webb 22) Filed: Apr. 13, 1984 57 ABSTRACT An earth battery which includes a first electrode which Related U.S. Application Data is a veined material located in the earth, a second elec 62) Division of Ser. No. 384,781, Jun. 4, 1982, Pat. No. trode electrically connected to the surface of the earth 4,457,988. and disposed in a hole which extends from the surface 51 Int. Cl." ....................... E21C 41/06; E21C 41/14 of the earth into the first electrode, an electrolyte dis 52) U.S. C. ........................................ 299/5; 166/248; posed in the hole and in contact with the first and sec 299/7; 299/8 ond electrodes, and means for electrically connecting 58) Field of Search ............................... 299/7, 8, 5, 4; the first electrode to the surface of the earth. A seam of 166/248, 65 R; 429/47, 13, 10 coal is preferred as the first electrode. A method of mining metals located in the earth using the earth bat 56 References Cited tery and a method of drilling holes in the earth are also U.S. -

Reliable Energy Sources As a Foundation for Reliable Intermittent Systems
Reliable Energy Sources as a Foundation for Reliable Intermittent Systems Dhananjay Jagtap Pat Pannuto [email protected] [email protected] University of California, San Diego University of California, San Diego ABSTRACT be accessible to change batteries [11]. This is the case when de- This paper defines architectural and operational principles for sim- ploying sensor networks for structural or condition monitoring ple and reliable energy harvesting devices that can be used in service in industrial environments [38] or for environmental monitoring of high-level applications. For many maintenance and monitoring applications where there is no built infrastructure. This is part of tasks, we propose that it is more valuable to have reliable, trusted what makes energy harvesting designs so exciting: they present affirmations that nothing has changed than it is to know theexact the potential for set-it-and-forget it systems that can go anywhere moment that something has failed. This presents an opportunity for and last forever [16]. a new class of highly reliable, but not necessarily timely, intermit- What often makes energy harvesting designs challenging, how- tent devices. These are devices that are capable of sending messages ever, is that energy sources are unreliable, unpredictable, or un- at reasonable, fixed intervals (e.g. once an hour or once a day). They controllable. Early designs simply ran whenever they could for as cannot activate more often, but they also promise not to activate long as they could [10, 31, 35]. However, this wholly uncontrolled less often. To establish this reliability, we look to opportunities for intermittency made it difficult for device and system designers energy scavenging that are often ignored as their instantaneous to build applications. -

Batteries and Primitive Survival E-Book
Batteries and Primitive Survival (An e-Book that is word searchable and printable) (3 Nov 2008) Table of Contents Introduction: Knowledge from many sources including my own research and development have been brought together to show how to intelligently use battery storage technology in a long term primitive survival situation. Lead-Acid batteries for use as primary source storage and smaller rechargeable/non-rechargeables for portability will be viewed in many different ways for practicality during primitive survival. The way to read this book is to read chapter one then jump to areas of interest, realizing that the proper storage of the correct chosen batteries and the making of efficient chargers are ideally needed before the emergency times start. This book is designed to be a reference work and is dedicated to those who wish to survive the coming pole shift. (by Mikel) Chapter 1: Basic Battery Survival 101 (7 pages) This chapter gives an overview summery of the basics knowledge needed to start one into primitive survival using storage cells and batteries. The remaining chapters detail and answer the questions needed to help survive in a primitive environment for an extended time. Chapter 2: Long Term Battery Storage and Self Discharge Rates (12 pages) This chapter details how to prepare batteries for long term storage. What are the choices of batteries and how to store them for the long term. The manufactures recommendations are detailed. A description of what to do as you take them out of storage for the first time is also detailed. Chapter 3: Efficient Battery Chargers for Survival (20 pages) Most of the currently efficient commercial battery chargers that I have tested have a maximum efficiency of about 20-38% of source energy delivered to the destination battery. -

1875 Patent on Earth Batteries
1875 Patent on Earth Batteries There has recently been a question on Earth batteries on the Free energy newsgroup as so many were unaware of the existence of such a device and must admit that I was ignorant of the device myself until I came across this patent and so I reproduce an except from the patent application below. "The object of my invention is to produce a current of electricity from an earth battery or batteries capable of generating a constant current of considerable intensity to be used for lightning rod and other purposes where voltaic batteries using solutions are now applied. It is known that if different elements-for instance sheets of zinc and copper-be buried or placed in the earth ,a current of electricity is generated; but I have discovered that if such elements be partly embedded in sulphur so that the dampness of the earth may act in conjunction with the sulphur on the metals, a more intense will be created. I utilise this in the following way: The current is collected by insulated wires coiled around nickel plated steel magnets ,which are planted north and south in the earth to receive the magnetic current of the earth; a secondary coil or coils of insulated wire surrounds the coil or coils around the magnets and receives by induction, electricity from both the voltaic and magneto-electro batteries. In the drawing, the voltaic battery is composed of several pieces or plates of chemically pure zinc B, and the same number of copper, A they are embedded in a cake of sulphur C and are connected by a large insulated wire D, which being the primary coil between dissimilar elements is extended, without insulation to the base of the sulphur cake C, and also in a spiral coil or coils around steel magnets E, which are pointed magnetised and nickel plated. -

Earth Clock Earth Clock
WARNING This is not a toy. It is an educational kit and must be used under adult supervision. This product is not suitable for children under 3 years. It contains sharp or small parts that could be dangerous Ca!re for young children. Needed EEaartrthh Manufactured and Supplied by Mindsets Copyright © Mindsets (UK) Limited, 2011 The Earth Clock is one of a number of innovative educational products designed at CCloclockk Middlesex University to interest young people in science and technology. Please ask your supplier for more details. Earth clock What to do next Welcome to the fascinating world of timekeeping. You have just Soil is an ideal material for your earth clock, but it must be wet. You bought a high-tech electronic clock that gets all of its power could run the clock from a pot plant but the pairs of metal strips from ordinary earth! You will never need expensive batteries – must be at least 5cm apart. If the soil gets too dry, the clock stops and you will be able to run your clock using other unlikely working and tells you that the plant must be watered. materials. Any other material that is slightly acidic can be made into a battery Avoiding the use of batteries is environmentally friendly. All the for your clock. Newspapers and cheap books use paper that has a energy and materials that go into the battery are saved, and high acid content. That is why they go brown over time. The acid there is nothing to dispose of when the battery is used attacks the paper. -

Electrohorticult00hullrich.Pdf
X* LIBRARY UNIVERSITY OF CALIFORNIA GIFT OK /9oo Received J&/VU Accession No. "YZO o 3 ' ^ass ^' / n I v/ \LSJ-. I&AM. ^ui<A <& <J . ELECTRO-HORTICULTURE BY GEO. S. HULL, M.D., Sc.D. PASADENA GAL. TEbe Knickerbocker jprcss i?ork COPYRIGHT, 1898 BY GEORGE S. HULL 7*03-3 PREFACE is analogous to heat and light, ELECTRICITYand, like them, has an influence upon the growth of plants. What this is, it is the purpose of the author to inquire into with his readers by presenting to them a summary of what has been accomplished in the comparatively new science of electro-horticulture, and by discussing with them the rationale of the action of electricity upon vegetation. That he may afford assistance to some who are already at work, and possibly influence others to investigate into this fascinating subject, is his sole motive in offering to the public this monograph. He has had in view the popular rather than the scientific aspect of the subject, and hence has limited himself to what he felt would most appeal to the average reader. G. S. II. PASADENA, CAL., October i, 1898. CONTENTS I. The Dawn of Electro-horticulture. The Appli- cation of Electricity to the Stalks of Plants . I II. Electricity from the Atmosphere Applied to the Roots of Plants and to the Soil Surrounding them ........ 8 IIT. Electricity from Batteries Applied to the Roots to . of Plants and the Surrounding Soil . 14 Effects Electric IV. of the Light upon Vegetation . 19 V. How Does Electricity Act upon Vegetation ? . 24 VI. -

Instructables.Com
Home Sign Up! Explore Community Submit All Art Craft Food Games Green Home Kids Life Music Offbeat Outdoors Pets Ride Science Sports Tech Building a "Baghdad Battery" by IanW on October 31, 2008 Table of Contents intro: Building a "Baghdad Battery" . 2 step 1: Construction Overview an Materials . 3 step 2: Making Fe3O4 for the Iron Electrode . 3 step 3: Constructing the Electrode Pairs . 4 step 4: Containment of Electrochemical Cells an Other Notes . 6 step 5: Monitoring Performance of Cells . 9 step 6: Performance of this "Baghdad Battery" replica . 11 step 7: How this 'replica' differs from other attempts . 14 step 8: Interpretation of the Artifacts . 17 step 9: History . 18 step 10: On other published accounts . 19 Related Instructables . 21 Advertisements . 21 Customized Instructable T-shirts . 21 Comments . 21 http://www.instructables.com/id/Building_a_quotBaghdad_Batteryquot/ intro: Building a "Baghdad Battery" The intent of this instructable is to provide ideas for experimenting with battery chemistry in general, and to summarize my experience producing a replica of what is often referred to as the Baghdad Battery. In my version of a replica I argue the artifacts have been misinterpreted in replicas previously built by other investigators. The replica I've created yields not only a better performing cell (capable of 5x the capacity), but one that is rechargeable. This is not a great cell, and our cars and homes will likely never employ the design, but it is curious that ~2000 years ago people may have been experimenting with electricity. What they were using this electricity for is subject to as much speculation as the actual construction of the "battery" (Perhaps it was not a battery, that is for you to decide). -

Heroes of the Telegraph
Heroes of the Telegraph J. Munro Project Gutenberg's Etext of Heroes of the Telegraph by J. Munro Copyright laws are changing all over the world, be sure to check the copyright laws for your country before posting these files!! Please take a look at the important information in this header. We encourage you to keep this file on your own disk, keeping an electronic path open for the next readers. Do not remove this. **Welcome To The World of Free Plain Vanilla Electronic Texts** **Etexts Readable By Both Humans and By Computers, Since 1971** *These Etexts Prepared By Hundreds of Volunteers and Donations* Information on contacting Project Gutenberg to get Etexts, and further information is included below. We need your donations. Heroes of the Telegraph by J. Munro July, 1997 [Etext #979] Project Gutenberg's Etext of Heroes of the Telegraph by J. Munro ******This file should be named htgrf10.txt or htgrf10.zip****** Corrected EDITIONS of our etexts get a new NUMBER, htgrf11.txt. VERSIONS based on separate sources get new LETTER, htgrf10a.txt. We are now trying to release all our books one month in advance of the official release dates, for time for better editing. Please note: neither this list nor its contents are final till midnight of the last day of the month of any such announcement. The official release date of all Project Gutenberg Etexts is at Midnight, Central Time, of the last day of the stated month. A preliminary version may often be posted for suggestion, comment and editing by those who wish to do so. -

Battery According to the Data in the Previous Part
UNIVERSIDAD POLITÉCNICA DE CARTAGENA Escuela Técnica Superior de Ingeniería Industrial Hami Tianshan build solar energy storage project. TRABAJO FIN DE MÁSTER MÁSTER UNIVERSITARIO EN ENERGÍAS RENOVABLES Auto NIJIAT AIMAITI AIMAITI r: Direc Antonio J. Fernández tor: Cartagena, 20/02/2019 1 Abstract This master thesis consists of two parts and an additional part. in the first part, we analyze the geographical and climatic conditions of the project area. design the most suitable photovoltaic power generation and the required photovoltaic power generation equipment. In the second part, we calculate the required battery according to the data in the previous part. then analyze the function of the battery to select the most suitable battery. additional parts to design battery room and heating measures for protecting batteries and other power generation equipment. 2 Index Introduction................................................................................................................................ 1 History of solar energy................................................................................................................2 And other material......................................................................................................................4 Same models...............................................................................................................................4 Solar energy Capacity in the world.............................................................................................8 -

Climate Change, Security, Sensors
acoustics Article Climate Change, Security, Sensors Giovanni P. Gregori 1,2,3,4 1 IDASC—Istituto di Acustica e Sensoristica O. M. Corbino (CNR), 00133 Rome, Italy; [email protected] 2 IEVPC— International Earthquake and Volcano Prediction Center, Orlando, FL 32860, USA 3 IASCC—Institute for Advanced Studies in Climate Change, Aurora, CO 80014, USA 4 ISSO—International Seismic Safety Organization, Headquarters, 64031 Arsita (TE), Italy Received: 16 April 2020; Accepted: 29 June 2020; Published: 3 July 2020 Abstract: A concise threefold illustration is given: (i) of climate change on the gigayear (Ga) time scale through the nanosecond (nsec) time scale, (ii) of the role of the performance of solid materials, concerning both manmade and natural structures with reference to security, and (iii) of the exploitation of the electrostatic energy of the atmospheric electrical circuit—which is an enormous reservoir of natural “clean” energy. Several unfortunate misunderstandings are highlighted that bias the present generally agreed beliefs. The typical natural pace of the Earth’s “electrocardiogram”, ~27.4 Ma, is such that, at present, for the first time humankind must challenge an Earth’s “heartbeat”. A correct use of sensors is needed to get an efficient monitoring of the ongoing climate change. Both anthropic and natural drivers are to be considered. A brief reminder is given about sensors that ought to monitor solid materials—with application (i) to every kind of machinery, building, viaduct or bridge, vehicle, aircraft, rocket, etc. and (ii) for a correct (and unprecedented) monitoring of the electric field at ground, which is the prerequisite for the exploitation of the electrostatic energy of the atmosphere. -

Battery Life and How to Improve It
Battery Life and How To Improve It Battery and Energy Technologies Technologies Battery Life (and Death) Low Power Cells High Power Cells For product designers, an understanding of the factors affecting battery life is vitally important for managing both product Chargers & Charging performance and warranty liabilities particularly with high cost, high power batteries. Offer too low a warranty period and you won't Battery Management sell any batteries/products. Overestimate the battery lifetime and you could lose a fortune. Battery Testing Cell Chemistries FAQ That batteries have a finite life is due to occurrence of the unwanted chemical or physical changes to, or the loss of, the active materials of which Free Report they are made. Otherwise they would last indefinitely. These changes are usually irreversible and they affect the electrical performance of the cell. Buying Batteries in China Battery life can usually only be extended by preventing or reducing the cause of the unwanted parasitic chemical effects which occur in the cells. Choosing a Battery Some ways of improving battery life and hence reliability are considered below. How to Specify Batteries Battery cycle life is defined as the number of complete charge - discharge cycles a battery can perform before its nominal capacity falls below Sponsors 80% of its initial rated capacity. Lifetimes of 500 to 1200 cycles are typical. The actual ageing process results in a gradual reduction in capacity over time. When a cell reaches its specified lifetime it does not stop working suddenly. The ageing process continues at the same rate as before so that a cell whose capacity had fallen to 80% after 1000 cycles will probably continue working to perhaps 2000 cycles when its effective capacity will have fallen to 60% of its original capacity.