Battery Life and How to Improve It
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Power Supply Using Earth Battery and Solar Pael
ITSI Transactions on Electrical and Electronics Engineering (ITSI-TEEE) _______________________________________________________________________________________________ Power Supply Using Earth Battery 1Harish D, 2T S Dhanalakshmi, 3Gireesh H R 1,2,3RRCE, Bangalore electronic devices. They can also be considered to Abstract: In view of robust and cost effective use of this natural power technology by unskilled village consumers replace high voltage low current charging power most suitable combinations of the commonly available supplies or ionization power supplies. Like earth metals were selected for further detailed characteristic batteries the sea batteries also may be considered for studies. Combinations of Magnesium anode and Coke similar applications. However, air batteries can be used cathode: Zinc anode and Graphite, cathode: Aluminum for bulk power production and grid system operation [3]. anode and Carbon cathode; Zinc anode and Copper In view of global energy crisis to be caused by natural cathodes gave 2.05, 1.40, 1.10 and 0.9 volts per cell. Typical end of oil and gas within next 50 to60 years time [9-11], rated power of a single Zn-Cu cell was measured to be few it has become very important to look for alternative tens of microamperes. Small power electronic devices such energy sources to hold back the human race from as calculators, electronic watches, baby toys and cell phones and white light LEDs were operated on site. The engagement to a great energy war [12-13]. Although, voltage level was found to increase linearly by connecting uranium [14] and coal [9] would continue to exist for multiple earth battery cells in series like commercial lead few centuries but they cannot replace oil and gas despite acid battery. -

Primary Energy Use and Environmental Effects of Electric Vehicles
Article Primary Energy Use and Environmental Effects of Electric Vehicles Efstathios E. Michaelides Department of Engineering, TCU, Fort Worth, TX 76132, USA; [email protected] Abstract: The global market of electric vehicles has become one of the prime growth industries of the 21st century fueled by marketing efforts, which frequently assert that electric vehicles are “very efficient” and “produce no pollution.” This article uses thermodynamic analysis to determine the primary energy needs for the propulsion of electric vehicles and applies the energy/exergy trade-offs between hydrocarbons and electricity propulsion of road vehicles. The well-to-wheels efficiency of electric vehicles is comparable to that of vehicles with internal combustion engines. Heat transfer to or from the cabin of the vehicle is calculated to determine the additional energy for heating and air-conditioning needs, which must be supplied by the battery, and the reduction of the range of the vehicle. The article also determines the advantages of using fleets of electric vehicles to offset the problems of the “duck curve” that are caused by the higher utilization of wind and solar energy sources. The effects of the substitution of internal combustion road vehicles with electric vehicles on carbon dioxide emission avoidance are also examined for several national electricity grids. It is determined that grids, which use a high fraction of coal as their primary energy source, will actually increase the carbon dioxide emissions; while grids that use a high fraction of renewables and nuclear energy will significantly decrease their carbon dioxide emissions. Globally, the carbon dioxide emissions will decrease by approximately 16% with the introduction of electric vehicles. -
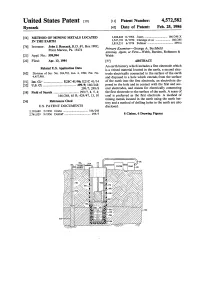
(1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb
United States Patent (19) (1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb. 25, 1986 54) METHOD OF MINING METALS LOCATED 3,288,648 11/1966 Jones ............................... 166/248 X N THE EARTH 3,547,192 12/1970 Claridge et al. .................... 166/248 3,819,231 6/1974 Fehliner ................................... 299/4 76 Inventor: John J. Ryeczek, R.D. #1, Box 190C, Primary Examiner-George A. Suchfield Point Marion, Pa. 15474 Attorney, Agent, or Firm-Webb, Burden, Robinson & 21 Appl. No.: 599,994 Webb 22) Filed: Apr. 13, 1984 57 ABSTRACT An earth battery which includes a first electrode which Related U.S. Application Data is a veined material located in the earth, a second elec 62) Division of Ser. No. 384,781, Jun. 4, 1982, Pat. No. trode electrically connected to the surface of the earth 4,457,988. and disposed in a hole which extends from the surface 51 Int. Cl." ....................... E21C 41/06; E21C 41/14 of the earth into the first electrode, an electrolyte dis 52) U.S. C. ........................................ 299/5; 166/248; posed in the hole and in contact with the first and sec 299/7; 299/8 ond electrodes, and means for electrically connecting 58) Field of Search ............................... 299/7, 8, 5, 4; the first electrode to the surface of the earth. A seam of 166/248, 65 R; 429/47, 13, 10 coal is preferred as the first electrode. A method of mining metals located in the earth using the earth bat 56 References Cited tery and a method of drilling holes in the earth are also U.S. -

A HISTORY of the SOLAR CELL, in PATENTS Karthik Kumar, Ph.D
A HISTORY OF THE SOLAR CELL, IN PATENTS Karthik Kumar, Ph.D., Finnegan, Henderson, Farabow, Garrett & Dunner, LLP 901 New York Avenue, N.W., Washington, D.C. 20001 [email protected] Member, Artificial Intelligence & Other Emerging Technologies Committee Intellectual Property Owners Association 1501 M St. N.W., Suite 1150, Washington, D.C. 20005 [email protected] Introduction Solar cell technology has seen exponential growth over the last two decades. It has evolved from serving small-scale niche applications to being considered a mainstream energy source. For example, worldwide solar photovoltaic capacity had grown to 512 Gigawatts by the end of 2018 (representing 27% growth from 2017)1. In 1956, solar panels cost roughly $300 per watt. By 1975, that figure had dropped to just over $100 a watt. Today, a solar panel can cost as little as $0.50 a watt. Several countries are edging towards double-digit contribution to their electricity needs from solar technology, a trend that by most accounts is forecast to continue into the foreseeable future. This exponential adoption has been made possible by 180 years of continuing technological innovation in this industry. Aided by patent protection, this centuries-long technological innovation has steadily improved solar energy conversion efficiency while lowering volume production costs. That history is also littered with the names of some of the foremost scientists and engineers to walk this earth. In this article, we review that history, as captured in the patents filed contemporaneously with the technological innovation. 1 Wiki-Solar, Utility-scale solar in 2018: Still growing thanks to Australia and other later entrants, https://wiki-solar.org/library/public/190314_Utility-scale_solar_in_2018.pdf (Mar. -

Solar Cells from Selenium to Sihcon: Powering the Future. Part 2 by C M Meyer, Technical Journalist
GENERATION Solar cells from selenium to sihcon: Powering the future. Part 2 by C M Meyer, technical journalist An amazingly simple device, capable of performing efficiently nearly all the functions of an ordinary vacuum tube, was demonstrated for the first time yesterday at Bell Telephone Laboratories where it was invented. Known as the transistor, the device works on an entirely new physical principle discovered by the laboratories in the course of fundamental research into the electrical properties of solids.” Press release from Bell Telephone Laboratories announcing the first transistor, 1 July 1948 (Ref.6). The very first photoelectric cell was a pretty crude device. It was basically a large, thin layer of selenium that had been spread onto a copper metal plate, and covered with a thin, semitransparent gold-leaf film. Even by February 1953, the efficiency of such selenium cells was not very high: only a little less than 0,5%. Small wonder then that a more efficient substitute was needed. So it is hardly surprising that scientists working for Bell Telephone Laboratory, many on commercialising the newly discovered transistor, were aware of this need. One of them, Daryl Chapin, had begun work on the problem of providing small amounts of intermittent power in remote humid locations. His research originally had nothing to do with solar power, and involved wind machines, thermoelectric generators and small steam engines. At his suggestion, solar power was included in his research, almost as an afterthought. After obtaining disappointing results with a The first attempt to launch a satellite with the Vanguard rocket on 6 December 1957. -

Bob Pease, Analog Guru, Died 5 Years Ago - Media Rako.Com/Media 1 of 12
Bob Pease, analog guru, died 5 years ago - Media Rako.com/Media 1 of 12 Rako Studios » Media » Tech » Electronics » Bob Pease, analog guru, died 5 years ago Bob Pease, analog guru, died 5 years ago Bob Pease was a true standout, the gentleman genius. Notorious analog engineer Bob Pease died five Saturday, Bob had come to the service from years ago, on June 18, 2011. His passing was his office at National Semiconductor, now all the more tragic since he died driving home Texas Instruments. My buddy has a from a remembrance for fellow analog saying, "Everyone wants to be somebody, no great Jim Williams. Although it was a one wants to become somebody." 1 of 12 9/2/2018 6:49 PM 1 of 12 Bob Pease, analog guru, died 5 years ago - Media Rako.com/Media 2 of 12 Bob's being the most famous analog designer After coming to National Semi, Bob learned was the result of his hard work becoming a analog IC design, back in the days ofhand-cut brilliant engineer, with a passion for helping Rubylith masks. There was no Spice simulator others. Fran Hoffart, retired Linear Tech apps back then, and Pease had deep ridicule for engineer and former colleague recalls, "Citing a engineers that relied on computer simulations, need for educating fellow engineers in the insteadof thinking through the problem and design of bandgap references, Bob anointed making some quickback-of-envelope himself 'The Czar of Bandgaps,' complete with calculations. He accepted that Spice was useful a quasi-military suit with a sword and a especially for inexperienced engineers, but was necklace made from metal TO-3 packages." He concerned thatengineers were substituting would help any engineer with a problem, even computer smarts for real smarts. -

Electric Battery Car Competition Rules
Colorado Middle School Car Competition Electric Battery Division The Colorado Middle School Car Competition is a classroom-based, hands-on educational program for 6th – 8th grade students. Student teams apply math, science, and creativity to construct and race model lithium-ion powered cars. The primary goals of the programs are to: • Generate enthusiasm for science and engineering at a crucial stage in the educational development of young people; • Improve students' understanding of scientific concepts and renewable energy technologies; and • Encourage young people to consider technical careers at an early age. Program description: • Students use mathematics and science principles together with their creativity in a fun, hands-on educational program. • Using engineering principles, students get excited about generating ideas in a group and then building and modifying models based on these ideas. • Students can see for themselves how changes in design are reflected in car performance. • Students work together on teams to apply problem solving and project management skills. The car competition challenges students to use scientific know-how, creative thinking, experimentation, and teamwork to design and build high-performance model electric battery vehicles. Rules Competition Structure: The Colorado competition will use preliminary time trials before progressing to a double elimination tournament for the finals. Each team will have three time trials to achieve their fastest time. Any car that does not finish in 40 seconds will be considered a Did Not Finish (DNF). Only the fastest 16 teams will progress to the double elimination tournament. In the event of a tie, teams will have a race-off to qualify for the double elimination round. -

Understanding the Cavity Duplexer
i THE CAVITY DUPLEXER John E Portune W6NBC [email protected] rev 2019 NOTE FROM AUTHOR This book was written several years ago and based on hardware-store copper water pipe as the source of home-brew duplexer construction materials. Later I began making from spun-aluminum commercial cake pans. Both require no welding. Unfortunately the price of copper is today much higher. Cake pans, however, are still reasonably priced, readily available and very acceptable as the basis especially for VHF cavities. Because of maximum cavity size limit, copper water pipe may still be indicated for UHF and above. In any case, how a duplexer operates is basic physics. No matter what the material, or whether the duplexer is commercial or home brew, the principles herein are universal to duplexer construction, modification and tuning. This book, however, is not finished. Repeater building is no longer my primary interest in ham radio. Some subjects could be added. But as it contains the essentials, I have placed on the internet incomplete. If you reproduce it, be so kind as to give proper author’s credits. W6NBC January 2019 . ii CHAPTER OUTLINE 1. The Mysterious Duplexer • The black box everybody uses but nobody understands • Keys to understanding it • This is not a cookbook 2. Let’s Make a Cavity • Home-brew 2M aluminum cavity • Example for the entire book • The best way to learn 3. Cavities • Mechanical and electrical properties of cavities • Basic structure of a duplexer • Why use cavities • Getting energy in and out: loops, probes, taps and ports • Three cavity types: Bp, Br, Bp/Br • Creating the other types • Helical resonators for 6M and 10M duplexers 4. -

Analog Father.Indd
The Father of Analog Integrated Circuits: Robert J. Widlar From: Tales of the Continuum: A Subsampled History of Analog Circuits By Thomas H. Lee, Center for Integrated Systems, Stanford University At a time when even discrete solid-state op-amps had not yet succeeded in displacing their vacuum tube coun- terparts, and the very value of the integrated circuit idea was still a legitimate topic of debate, Bob Widlar (“wide-lar”) almost single-handedly established the discipline of analog IC design. After receiving his bach- elor’s degree in 1962 from the University of Colorado at Boulder, he took a job with Ball Brothers Research, where his virtuosity at circuit design attracted the attention of engineers at one of their components suppliers. Despite the breach in protocol inherent in aggressively recruiting a customer’s key employee, Fairchild induced Widlar to leave Ball in late 1963. In an amazing debut, abetted by Dave Talbert’s brilliant process engineering, Widlar was able to put the world’s first integrated circuit op-amp into production by 1964. Development of the μA702, as Fairchild called it, proceeded despite a general lack of enthusiasm for the project at the company. The Fairchild μA702 Like the K2-W, this op-amp consists of two primary voltage gain stages (Figure 2). As in most differential designs, there is the problem of how to convert to a single-ended output without sacrificing half of the gain (the K2-W simply makes that sacrifice). Here the young Widlar solves this problem with a circuit that presages his later use of current-mirror loads. -

Mos Technology, 1963-1974: a Dozen Crucial Years
One of IBM’s most important MOS Technology, 1963-1974: A Dozen Crucial Years contributions to MOS research came from the Components Division, which was responsible for developing by Ross Knox Bassett and manufacturing bipolar transistors for its large computer systems and had very little interest in MOS transistors line can be drawn from the as such. As part of its work on Frosch’s and Derick’s work on bipolar transistors, Donald Kerr and silicon dioxide to the MOS (metal- a group of engineers had discovered A that depositing small amounts of oxide-semiconductor) transistor’s domi- nance of semiconductor technology, phosphorous on the silicon-dioxide but it is neither short nor straight. That surface and forming a layer of line has several discernable segments, phosphosilicate glass (PSG) could first from Frosch and Derick’s work, limit the amount of leakage in bipolar until 1963. In this interval, by and transistors and play an important role large, no one thought seriously about a in enhancing the stability of MOS metal-oxide-semiconductor as a viable transistors. Jerome Eldridge and Pieter technology in its own right. The second Balk from IBM Research implemented segment runs from 1963, when the this work by using thin layers of combination of integrated circuits and PSG to make stable MOS devices. the planar manufacturing process had Other important work on the physics FIG. 2. Drawing of Atalla and Kahng’s “silicon-silicon dioxide surface device,” now known as and chemistry of MOS devices done led people to see MOS transistors as a the MOS transistor, from a 1961 Bell Labs technical memorandum by Kahng. -

A „Szőke Tisza” Megmentésének Lehetőségei
A „SZŐKE TISZA” MEGMENTÉSÉNEK LEHETŐSÉGEI Tájékoztató Szentistványi Istvánnak, a szegedi Városkép- és Környezetvédelmi Bizottság elnökének Összeállította: Dr. Balogh Tamás © 2012.03.27. TIT – Hajózástörténeti, -Modellező és Hagyományőrző Egyesület 2 TÁJÉKOZTATÓ Szentistványi István, a szegedi Városkép- és Környezetvédelmi Bizottság elnöke részére a SZŐKE TISZA II. termesgőzössel kapcsolatban 2012. március 27-én Szentistványi István a szegedi Városkép- és Környezetvédelmi Bizottság elnöke e-mailben kért tájékoztatást Dr. Balogh Tamástól a TIT – Hajózástörténeti, -Modellező és Hagyományőrző Egyesület elnökétől a SZŐKE TISZA II. termesgőzössel kapcsolatban, hogy tájékozódjon a hajó megmentésének lehetőségéről – „akár jelentősebb anyagi ráfordítással, esetleges városi összefogással is”. A megkeresésre az alábbi tájékoztatást adom: A hajó 2012. február 26-án süllyedt el. Azt követően egyesületünk honlapján – egy a hajónak szentelt tematikus aloldalon – rendszeresen tettük közzé a hajóra és a mentésére vonatkozó információkat, képeket, videókat (http://hajosnep.hu/#!/lapok/lap/szoke-tisza-karmentes), amelyekből szinte napi ütemezésben nyomon követhetők a február 26-március 18 között történt események. A honlapon elérhető információkat nem kívánom itt megismételni. Egyebekben a hajó jelentőségéről és az esetleges városi véleménynyilvánítás elősegítésére az alábbiakat tartom szükségesnek kiemelni: I) A hajó jelentősége: Bár a Kulturális Örökségvédelmi Hivatal előtt jelenleg zajlik a hajó örökségi védelembe vételére irányuló eljárás (a hajó örökségi -

Deep Cycle Battery Frequently Asked Questions
DEEP CYCLE BATTERY FREQUENTLY ASKED QUESTIONS DEEP CYCLE BATTERY FAQ The links below are on this page - you can also just scroll down if you want to read them all. This entire page is copyright 1998-2014 by Northern Arizona Wind & Sun. Please do not use without prior permission. • What is a Battery? • Types of Batteries • Battery Lifespan • Starting, Marine, or Deep Cycle? • Deep Cycle Battery as a Starting Battery? • What Batteries are made of • Industrial Deep Cycle Batteries (fork lift type) • Sealed Batteries • Battery Size Codes • Gel Cells (Gelled Electrolyte) (and why we don't like them) • AGM Batteries (and why we do like them) • Temperature Effects • Cycles vs Lifespan • Amp-Hours - what are they? • Battery Voltages • Battery Charging (Here is where we get into the real meat) • Charge Controllers (for wind/solar) • Mini Factoids - Some small facts about batteries The subject of batteries could take up many pages. All we have room for here is a basic overview of batteries commonly used with photovoltaic power systems. These are nearly all various variations of Lead-Acid batteries. For a very brief discussion on the advantages and disadvantages of these and other types of batteries, such as NiCad, NiFe (Nickel-Iron), etc. go to our Batteries for Deep Cycle Applications page. These are sometimes referred to as "deep discharge" or "deep cell" batteries. The correct term is deep cycle. A printable version of this page will be available in Adobe PDF format when we finish updating this page for downloading and printing: Most of the charts have small images for faster downloading.