Battery According to the Data in the Previous Part
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Power Supply Using Earth Battery and Solar Pael
ITSI Transactions on Electrical and Electronics Engineering (ITSI-TEEE) _______________________________________________________________________________________________ Power Supply Using Earth Battery 1Harish D, 2T S Dhanalakshmi, 3Gireesh H R 1,2,3RRCE, Bangalore electronic devices. They can also be considered to Abstract: In view of robust and cost effective use of this natural power technology by unskilled village consumers replace high voltage low current charging power most suitable combinations of the commonly available supplies or ionization power supplies. Like earth metals were selected for further detailed characteristic batteries the sea batteries also may be considered for studies. Combinations of Magnesium anode and Coke similar applications. However, air batteries can be used cathode: Zinc anode and Graphite, cathode: Aluminum for bulk power production and grid system operation [3]. anode and Carbon cathode; Zinc anode and Copper In view of global energy crisis to be caused by natural cathodes gave 2.05, 1.40, 1.10 and 0.9 volts per cell. Typical end of oil and gas within next 50 to60 years time [9-11], rated power of a single Zn-Cu cell was measured to be few it has become very important to look for alternative tens of microamperes. Small power electronic devices such energy sources to hold back the human race from as calculators, electronic watches, baby toys and cell phones and white light LEDs were operated on site. The engagement to a great energy war [12-13]. Although, voltage level was found to increase linearly by connecting uranium [14] and coal [9] would continue to exist for multiple earth battery cells in series like commercial lead few centuries but they cannot replace oil and gas despite acid battery. -
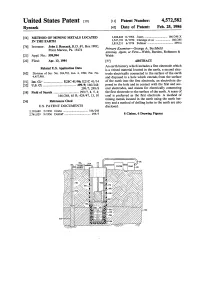
(1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb
United States Patent (19) (1) Patent Number: 4,572,582 Ryeczek 45 Date of Patent: Feb. 25, 1986 54) METHOD OF MINING METALS LOCATED 3,288,648 11/1966 Jones ............................... 166/248 X N THE EARTH 3,547,192 12/1970 Claridge et al. .................... 166/248 3,819,231 6/1974 Fehliner ................................... 299/4 76 Inventor: John J. Ryeczek, R.D. #1, Box 190C, Primary Examiner-George A. Suchfield Point Marion, Pa. 15474 Attorney, Agent, or Firm-Webb, Burden, Robinson & 21 Appl. No.: 599,994 Webb 22) Filed: Apr. 13, 1984 57 ABSTRACT An earth battery which includes a first electrode which Related U.S. Application Data is a veined material located in the earth, a second elec 62) Division of Ser. No. 384,781, Jun. 4, 1982, Pat. No. trode electrically connected to the surface of the earth 4,457,988. and disposed in a hole which extends from the surface 51 Int. Cl." ....................... E21C 41/06; E21C 41/14 of the earth into the first electrode, an electrolyte dis 52) U.S. C. ........................................ 299/5; 166/248; posed in the hole and in contact with the first and sec 299/7; 299/8 ond electrodes, and means for electrically connecting 58) Field of Search ............................... 299/7, 8, 5, 4; the first electrode to the surface of the earth. A seam of 166/248, 65 R; 429/47, 13, 10 coal is preferred as the first electrode. A method of mining metals located in the earth using the earth bat 56 References Cited tery and a method of drilling holes in the earth are also U.S. -

ECS Classics: Weston, the Weston Cell, and the Volt
ClassicsECS Weston, the Weston Cell, and the Volt by Petr Vanýsek he measurement of electromotive force, potential, or voltage Sharing much of the same enthusiasm as Acheson (ECS Classics, difference has been central to measurements ever since the Interface, 26(1) 36-39), Edison, or Swann (ECS Classics, Interface, Tconcept of potential in electricity was first understood. The 23(4) 38-40), Weston was also interested in lighting equipment. His definition of the volt changed a few times throughout the course of company, among others, won the contract to illuminate the new history and at one point it was even based on electrochemistry, on Brooklyn Bridge. His carbon based light bulb filament made from the so-called Clark cell. The international volt was defined in 1893 Tamidine (reduced nitrocellulose) was used until tungsten was as 1/1.434 electromotive force of the Clark cell. This definition lasted introduced. until 1908. The Clark cells used zinc or zinc amalgam for the anode and Weston was, since his first introduction to electrochemistry in mercury in a saturated aqueous solution of zinc sulfate for a cathode, electroplating, well aware of the need to reliably measure electrical with a paste of mercurous sulfate as a depolarizer. The cell design parameters. Because of this interest, in 1887 he established a had a drawback in a rather significant temperature coefficient and also laboratory making devices for measuring electrical parameters. In the suffered corrosion problems, which were caused by platinum wires process he developed two important alloys, constantan and manganin, that were alloying with the zinc amalgam in the glass envelope. -

Reliable Energy Sources As a Foundation for Reliable Intermittent Systems
Reliable Energy Sources as a Foundation for Reliable Intermittent Systems Dhananjay Jagtap Pat Pannuto [email protected] [email protected] University of California, San Diego University of California, San Diego ABSTRACT be accessible to change batteries [11]. This is the case when de- This paper defines architectural and operational principles for sim- ploying sensor networks for structural or condition monitoring ple and reliable energy harvesting devices that can be used in service in industrial environments [38] or for environmental monitoring of high-level applications. For many maintenance and monitoring applications where there is no built infrastructure. This is part of tasks, we propose that it is more valuable to have reliable, trusted what makes energy harvesting designs so exciting: they present affirmations that nothing has changed than it is to know theexact the potential for set-it-and-forget it systems that can go anywhere moment that something has failed. This presents an opportunity for and last forever [16]. a new class of highly reliable, but not necessarily timely, intermit- What often makes energy harvesting designs challenging, how- tent devices. These are devices that are capable of sending messages ever, is that energy sources are unreliable, unpredictable, or un- at reasonable, fixed intervals (e.g. once an hour or once a day). They controllable. Early designs simply ran whenever they could for as cannot activate more often, but they also promise not to activate long as they could [10, 31, 35]. However, this wholly uncontrolled less often. To establish this reliability, we look to opportunities for intermittency made it difficult for device and system designers energy scavenging that are often ignored as their instantaneous to build applications. -

Batteries and Primitive Survival E-Book
Batteries and Primitive Survival (An e-Book that is word searchable and printable) (3 Nov 2008) Table of Contents Introduction: Knowledge from many sources including my own research and development have been brought together to show how to intelligently use battery storage technology in a long term primitive survival situation. Lead-Acid batteries for use as primary source storage and smaller rechargeable/non-rechargeables for portability will be viewed in many different ways for practicality during primitive survival. The way to read this book is to read chapter one then jump to areas of interest, realizing that the proper storage of the correct chosen batteries and the making of efficient chargers are ideally needed before the emergency times start. This book is designed to be a reference work and is dedicated to those who wish to survive the coming pole shift. (by Mikel) Chapter 1: Basic Battery Survival 101 (7 pages) This chapter gives an overview summery of the basics knowledge needed to start one into primitive survival using storage cells and batteries. The remaining chapters detail and answer the questions needed to help survive in a primitive environment for an extended time. Chapter 2: Long Term Battery Storage and Self Discharge Rates (12 pages) This chapter details how to prepare batteries for long term storage. What are the choices of batteries and how to store them for the long term. The manufactures recommendations are detailed. A description of what to do as you take them out of storage for the first time is also detailed. Chapter 3: Efficient Battery Chargers for Survival (20 pages) Most of the currently efficient commercial battery chargers that I have tested have a maximum efficiency of about 20-38% of source energy delivered to the destination battery. -

Electronic Supplementary Information Electrosynthesis, Functional, And
Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2012 Electronic Supplementary Information Electrosynthesis, Functional, and Structural Characterization of a Water-Oxidizing Manganese Oxide Ivelina Zaharieva,*a Petko Chernev, a Marcel Risch,a, c Katharina Klingan,a Mike Kohlhoff,a Anna Fischer,b and Holger Dau*a a Fachbereich Physik, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. E-mail: [email protected]; [email protected]; Fax: +49 30 838 56299; Tel: +49 30 838 53581 b Institute of Chemistry, Technical University Berlin, Straße des 17. Juni, 10623 Berlin, Germany c present address: Electrochemical Energy Laboratory, MIT, 77 Massachusetts Ave, Cambridge, MA-02139, USA S1 Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2012 S-1. Details of experimental procedures A. Electrodeposition. Reagents: (Mn(CH3COO)2·4H2O (Fluka, ≥99%), H2KPO4 (Roth, ≥99%), K2HPO4 (Roth, ≥99%), MgSO4·7H2O (AppliChem, ≥99.5%), Na(CH3COO) (Sigma-Aldrich, ≥99%), CH3COOH (AppliChem, 100%), MnCl2·4H2O (Roth, ≥98%), Mg(CH3COO)2·4H2O (Fluka, 99%), MnSO4·4H2O (Merck, p.A.), NaClO4 (Aldrich, 99.99% trace metal basis), II II Co (OH2)6(NO3)2 (Sigma Aldrich, ≥99.9%), Ni (OH2)6(NO3)2 (ChemPur, ≥99%), H3BO3 (Merck, p.A.), KOH (Sigma Aldrich, ≥86%). All reagents where used without further purification. Solutions were prepared with 18 MΩ·cm Milli-Q water. The electrochemical experiments were performed at room temperature using a potentiostat (SP-300, BioLogic Science Instruments) controlled by EC-Lab v10.20 software package. The Mn films were deposited in de- ionised water, in 0.1 M MgSO4 or in 0.1 M Na acetate buffer (pH 6.0). -

1875 Patent on Earth Batteries
1875 Patent on Earth Batteries There has recently been a question on Earth batteries on the Free energy newsgroup as so many were unaware of the existence of such a device and must admit that I was ignorant of the device myself until I came across this patent and so I reproduce an except from the patent application below. "The object of my invention is to produce a current of electricity from an earth battery or batteries capable of generating a constant current of considerable intensity to be used for lightning rod and other purposes where voltaic batteries using solutions are now applied. It is known that if different elements-for instance sheets of zinc and copper-be buried or placed in the earth ,a current of electricity is generated; but I have discovered that if such elements be partly embedded in sulphur so that the dampness of the earth may act in conjunction with the sulphur on the metals, a more intense will be created. I utilise this in the following way: The current is collected by insulated wires coiled around nickel plated steel magnets ,which are planted north and south in the earth to receive the magnetic current of the earth; a secondary coil or coils of insulated wire surrounds the coil or coils around the magnets and receives by induction, electricity from both the voltaic and magneto-electro batteries. In the drawing, the voltaic battery is composed of several pieces or plates of chemically pure zinc B, and the same number of copper, A they are embedded in a cake of sulphur C and are connected by a large insulated wire D, which being the primary coil between dissimilar elements is extended, without insulation to the base of the sulphur cake C, and also in a spiral coil or coils around steel magnets E, which are pointed magnetised and nickel plated. -

I66 the CADMIUM STANDARD CELL. a STANDARD Cell with A
i66 G. A. HULETT. [VOL. XXIII. THE CADMIUM STANDARD CELL. BY GEORGE A. HULETT. A STANDARD cell with a low temperature coefficient was de- ^~*- vised by Mr. Edward Weston in 1891.1 By substituting cad mium sulphate and cadmium amalgam for the zinc sulphate and zinc of the Clark cell, it was found that the temperature coefficient of the cell was reduced 95 per cent., and when the solid cadmium sulphate was dispensed with and only a solution of cadmium sul phate used in the cell, the temperature coefficient was still less. With a solution saturated at o° C, Barnes and Lucas found2 that the E.M.F. of the cell was constant for all ordinary temperature changes. This latter combination is a valuable laboratory instru ment, but is not sufficiently reproducible or constant to serve as a standard of E.M.F. The Clark cell has a variation of E.M.F. of over a millivolt per degree, and for accurate work requires a very exact control of the temperature for a considerable time. The saturated cadmium cell has only one twentieth of the temperature coefficient of the Clark cell and many other desirable features, so that considerable work has been done in testing its reproducibility and constancy. A. Dearlove3 found that attention must be given to the cadmium amalgam and concluded that it was best to use a 12.5 per cent. amalgam, made by melting together one part of cadmium and seven parts of mercury. Since then considerable work has been done on cadmium amalgams. Kerp and Boettger4 concluded that the solid which separates from cadmium amalgams of over 5 per cent, was a compound represented by the formula Cd2Hgr However, from the work of Bijl5 and Puschin 6 it appears that between about 5 and 15 LU. -

An Examination of the Catalyst Layer Contribution to the Disparity Between the Nernst Potential and Open Circuit Potential in Proton Exchange Membrane Fuel Cells
catalysts Article An Examination of the Catalyst Layer Contribution to the Disparity between the Nernst Potential and Open Circuit Potential in Proton Exchange Membrane Fuel Cells Peter Mardle 1 , Isotta Cerri 2, Toshiyuki Suzuki 3 and Ahmad El-kharouf 1,* 1 School of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] 2 Toyota Motor Europe, Hoge Wei 33, 1930 Zaventem, Belgium; [email protected] 3 Toyota Motor Corporation, Toyota City 471-8571, Japan; [email protected] * Correspondence: [email protected]; Tel.: +44-121-414-5081 Abstract: The dependency of the Nernst potential in an operating proton exchange membrane fuel cell (PEMFC) on the temperature, inlet pressure and relative humidity (RH) is examined, highlighting the synergistic dependence of measured open circuit potential (OCP) on all three parameters. An alternative model of the Nernst equation is derived to more appropriately represent the PEMFC system where reactant concentration is instead considered as the activity. Ex situ gas diffusion electrode (GDE) measurements are used to examine the dependency of temperature, electrolyte concentration, catalyst surface area and composition on the measured OCP in the absence of H2 crossover. This is supported by single-cell OCP measurements, wherein RH was also investigated. This contribution provides clarity on the parameters that affect the practically measured OCP as well Citation: Mardle, P.; Cerri, I.; Suzuki, as highlighting further studies into the effects of catalyst particle surrounding environment on OCP T.; El-kharouf, A. An Examination of as a promising way of improving PEMFC performance in the low current density regime. -

Dissolved Oxygen Measurement
Dissolved Oxygen Measurement Introduction • Electrolyte - The electrolyte facilitates dissolved oxygen migration and provides an electrical path Dissolved oxygen is defined as the measure of water to complete the current loop. It also removes metal quality indicating free oxygen dissolved in water. The oxides (a by-product of the reaction) from the quantity of dissolved oxygen in water is typically electrodes so that their metal surfaces are clean to expressed in parts per million (ppm) or milligrams per react. Electrolyte must be periodically replenished liter (mg/l). Since oxygen is soluble in water, the to insure that the electrodes remain clean. amount of dissolved oxygen in water is in the state of dynamic equilibrium. The solubility of the dissolved The operational theory of a membrane sensor is that oxygen is proportional to the temperature and oxygen in the wastewater diffuses through the pressure of the water. membrane into the electrolyte. The concentration of gases always tends to equalize on both sides of the The most common application for dissolved oxygen membrane. When the concentration is not equal, gas measurement occurs in wastewater treatment. molecules migrate to the membrane side that has a Biochemical breakdown of sewage is achieved by lower concentration. When the membrane is bacterial attack in the presence of oxygen. This functioning, dissolved oxygen concentration in the process typically takes place in an aeration basin of a electrolyte in the measurement cell approximately wastewater treatment plant, and is accomplished by equals the dissolved oxygen concentration of the aerating or bubbling air (or pure oxygen) through the wastewater contacting the opposite side of the wastewater. -

A Convenient Standard Cell
Proceedings of the Iowa Academy of Science Volume 22 Annual Issue Article 23 1915 A Convenient Standard Cell Dieu Ung Huong State University of Iowa J. N. Pearce State University of Iowa Let us know how access to this document benefits ouy Copyright ©1915 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Huong, Dieu Ung and Pearce, J. N. (1915) "A Convenient Standard Cell," Proceedings of the Iowa Academy of Science, 22(1), 169-174. Available at: https://scholarworks.uni.edu/pias/vol22/iss1/23 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Huong and Pearce: A Convenient Standard Cell CONVENIENT STANDARD CELL 169 A CONVENIENT STANDARD CELL. DIED UNG HUONG AND J. N. PEARCE. Until receptly both the Clark and the Weston cells have served as standard sources of electromotive force. Both of these con sist ofl an amalgam of a metal as the anode covered by a satur ated solution of the sulphate of the metal and this in conjunc tion with mercury and mercurous sulphate which serves as the cathode. Clark cell: (Hg-Zn)-ZnS04-Hg2SO,-Hg. Weston cell: (Hg-Cd)-CdSO,-Hg2SO,-Hg. For various reasons the acceptance of the Clark cell as a standard has been discontinued. The Weston cell, chiefly on account of its approximately negligible temperature coefficient, is now the sole accepted standard of electromotive force. -

Water Quality Procedures
WAB Field SOP 2018 Revision Date: 8/22/2018 CHAPTER 3. WATER COLLECTION PROTOCOLS Overview A sonde is a collection of multiple sensors and probes housed into one casing and designed to give readings on multiple parameters simultaneously (often referred to as a Multi-Parameter Sonde). Sondes can vary in length, diameter, and housing construction. The most common four sensors on sondes are Temperature, pH, Dissolved Oxygen, and Specific Conductance (typically housed in a 2-inch diameter casing). Larger sondes (e.g., 3.5 – 4-inch diameter casing) can be equipped with additional, larger sensors and probes (e.g., Depth, Chlorophyll-a, and Turbidity). On some sondes (e.g., YSI models) the sensors and probes can easily be changed or replaced by the user. On other sondes (e.g., Hydrolab models), the sensors and probes cannot be easily changed without opening the sonde housing and electronics (either by a very experienced user or by sending the instrument back to the manufacturer’s service department). Figure 3-1. Example of a YSI 660 XL Sonde (right) and a 650 MDS Display Unit (left). Sensors and probes may utilize drastically contrasting technologies to measure a parameter. For example, one sonde may have a Dissolved Oxygen sensor that utilizes Water Collection-Overview Page | 3-1 WAB Field SOP 2018 Revision Date: 8/22/2018 the smaller Clark-cell technology (it has a membrane) with a stirrer; another sonde may use a similar Clark-cell technology and not have a stirrer; yet another sonde may use the larger Luminescence (LDO) technology (no membrane). In this case, the different sensor/probe technologies respectively offer pros and cons based on size the accuracy of the reading and size of the sonde casing required to house the sensor.