The Main Tea Eta a Cel Mai Te Rita Maria Matat An
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ECS Classics: Weston, the Weston Cell, and the Volt
ClassicsECS Weston, the Weston Cell, and the Volt by Petr Vanýsek he measurement of electromotive force, potential, or voltage Sharing much of the same enthusiasm as Acheson (ECS Classics, difference has been central to measurements ever since the Interface, 26(1) 36-39), Edison, or Swann (ECS Classics, Interface, Tconcept of potential in electricity was first understood. The 23(4) 38-40), Weston was also interested in lighting equipment. His definition of the volt changed a few times throughout the course of company, among others, won the contract to illuminate the new history and at one point it was even based on electrochemistry, on Brooklyn Bridge. His carbon based light bulb filament made from the so-called Clark cell. The international volt was defined in 1893 Tamidine (reduced nitrocellulose) was used until tungsten was as 1/1.434 electromotive force of the Clark cell. This definition lasted introduced. until 1908. The Clark cells used zinc or zinc amalgam for the anode and Weston was, since his first introduction to electrochemistry in mercury in a saturated aqueous solution of zinc sulfate for a cathode, electroplating, well aware of the need to reliably measure electrical with a paste of mercurous sulfate as a depolarizer. The cell design parameters. Because of this interest, in 1887 he established a had a drawback in a rather significant temperature coefficient and also laboratory making devices for measuring electrical parameters. In the suffered corrosion problems, which were caused by platinum wires process he developed two important alloys, constantan and manganin, that were alloying with the zinc amalgam in the glass envelope. -

The Silver Voltameter—Part Iv
.. 1 THE SILVER VOLTAMETER—PART IV THIRD SERIES OF QUANTITATIVE EXPERIMENTS AND SPECIAL INVESTIGATIONS By E. B. Rosa, G. W. Vinal, and A. S. McDanlel CONTENTS Page I. Introduction 476 II. DETERMINATION OP VOLTAGE OF THE WESTON NoRMAIv Cei.I< 477 A. Basis of reference for current and voltage measurements 478 1. Weston normal cells 478 2. Resistances 478 B. Meaning of the term " International volts" 478 C. Voltameters and materials 479 D. Tables of results 481 1. Corrections for acid present 482 2. Resultsof the porous pot and Smith's new-form voltameters. 485 3. Results of other forms of voltameters 492 4. Miscellaneous results 493 III. Speciai, Investigations 497 A. On the agreement of various forms 497 1 Comparative results 497 2 Possible explanations of observed differences 498 B. On the accuracy of the measurements. 501 C. Reliability of iodeosine as an indicator 502 D. Effect of acid 503 1 Effect on impure solutions 503 2 Nature of the action of acid 505 E. Influence of septa other than filter paper 508 1. Silk 508 2 Poroiis pots 510 3. Electrostenolysis 514 F. Purity of the silver deposit 516 1. Constancy of any error due to inclusions 516 2. Previous determinations of inclusions 517 3. Experiments on inclusions 519 (a) Detection of silver nitrate 519 (b) Estimation of included water and volatile material 52 (c) Estimation of organic material 522 (d) Titration of silver deposits 523 (e) Conclusion 523 475 476 Bulletin of the Bureau of Standards {Voi.io III. SpeciaIv Investigations—Continued. Page, G. Reversibility of the silver voltameter 523 H. -

Technical Report 91-39
TECHNICAL REPORT 91-39 The reductibility of sulphuric acid and sulphate in aqueous solution (translated from German) Rolf Grauer Paul Scherrer Institute, Switzerland July 1990 SVENSK KARNBRANSLEHANTERING AB SWEDISH NUCLEAR FUEL AND WASTE MANAGEMENT CO BOX 5864 S-102 48 STOCKHOLM TEL 08-665 28 00 TELEX 13108 SKB S TELEFAX 08-661 5719 THE REDUCIBILITY OF SULPHURIC ACID AND SULPHATE IN AQUEOUS SOLUTION (TRANSLATED FROM GERMAN) Rolf Grauer Paul Scherrer Institute, Switzerland July 1990 This report concerns a study which was conducted for SKB. The conclusions and viewpoints presented in the report are those of the author(s) and do not necessarily coincide with those of the client. Information on SKB technical reports from 1977-1978 (TR 121), 1979 (TR 79-28), 1980 (TR 80-26), 1981 (TR 81-17), 1982 (TR 82-28), 1983 (TR 83-77), 1984 (TR 85-01), 1985 (TR 85-20), 1986 (TR 86-31), 1987 (TR 87-33), 1988 (TR 88-32), 1989 (TR 89-40) and 1990 (TR 90-46) is available through SKB. Translation from German THE REDUCIBILITY OF SULPHURIC ACID AND SULPHATE IN AQUEOUS SOLUTION (fiber die Reduzierbarkeit von Schwefelsaure und Sulfat ir. wäBriger Lösung) by Rolf Grauer Paul Scherrer Institute July 1990 SUMMARY 1 1. INTRODUCTION 3 2. STATEMENT OF THE PROBLEM AND THE STRATEGY FOR SOLVING IT 4 3. THERMODYNAMICS OF CORROSION BY SULPHATE 5 4. GEOCHEMICAL INFORMATION ABOUT THE REDOX STABILITY OF SULPHATE 7 5. EXPERIMENTS ON THE REDUCTIBILITY OF SULPHURIC ACID AND SULPHATE 8 5.1 Hydrothermal experiments 8 5.2 The oxidising action of sulphuric acid on metals 9 5.3 Experiments on the cathodic reduction of sulphuric acid and sulphate 9 5.4 Polarographic investigations of the cathodic behaviour of sulphuric acid 10 6. -

Electronic Supplementary Information Electrosynthesis, Functional, And
Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2012 Electronic Supplementary Information Electrosynthesis, Functional, and Structural Characterization of a Water-Oxidizing Manganese Oxide Ivelina Zaharieva,*a Petko Chernev, a Marcel Risch,a, c Katharina Klingan,a Mike Kohlhoff,a Anna Fischer,b and Holger Dau*a a Fachbereich Physik, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. E-mail: [email protected]; [email protected]; Fax: +49 30 838 56299; Tel: +49 30 838 53581 b Institute of Chemistry, Technical University Berlin, Straße des 17. Juni, 10623 Berlin, Germany c present address: Electrochemical Energy Laboratory, MIT, 77 Massachusetts Ave, Cambridge, MA-02139, USA S1 Electronic Supplementary Material (ESI) for Energy & Environmental Science This journal is © The Royal Society of Chemistry 2012 S-1. Details of experimental procedures A. Electrodeposition. Reagents: (Mn(CH3COO)2·4H2O (Fluka, ≥99%), H2KPO4 (Roth, ≥99%), K2HPO4 (Roth, ≥99%), MgSO4·7H2O (AppliChem, ≥99.5%), Na(CH3COO) (Sigma-Aldrich, ≥99%), CH3COOH (AppliChem, 100%), MnCl2·4H2O (Roth, ≥98%), Mg(CH3COO)2·4H2O (Fluka, 99%), MnSO4·4H2O (Merck, p.A.), NaClO4 (Aldrich, 99.99% trace metal basis), II II Co (OH2)6(NO3)2 (Sigma Aldrich, ≥99.9%), Ni (OH2)6(NO3)2 (ChemPur, ≥99%), H3BO3 (Merck, p.A.), KOH (Sigma Aldrich, ≥86%). All reagents where used without further purification. Solutions were prepared with 18 MΩ·cm Milli-Q water. The electrochemical experiments were performed at room temperature using a potentiostat (SP-300, BioLogic Science Instruments) controlled by EC-Lab v10.20 software package. The Mn films were deposited in de- ionised water, in 0.1 M MgSO4 or in 0.1 M Na acetate buffer (pH 6.0). -

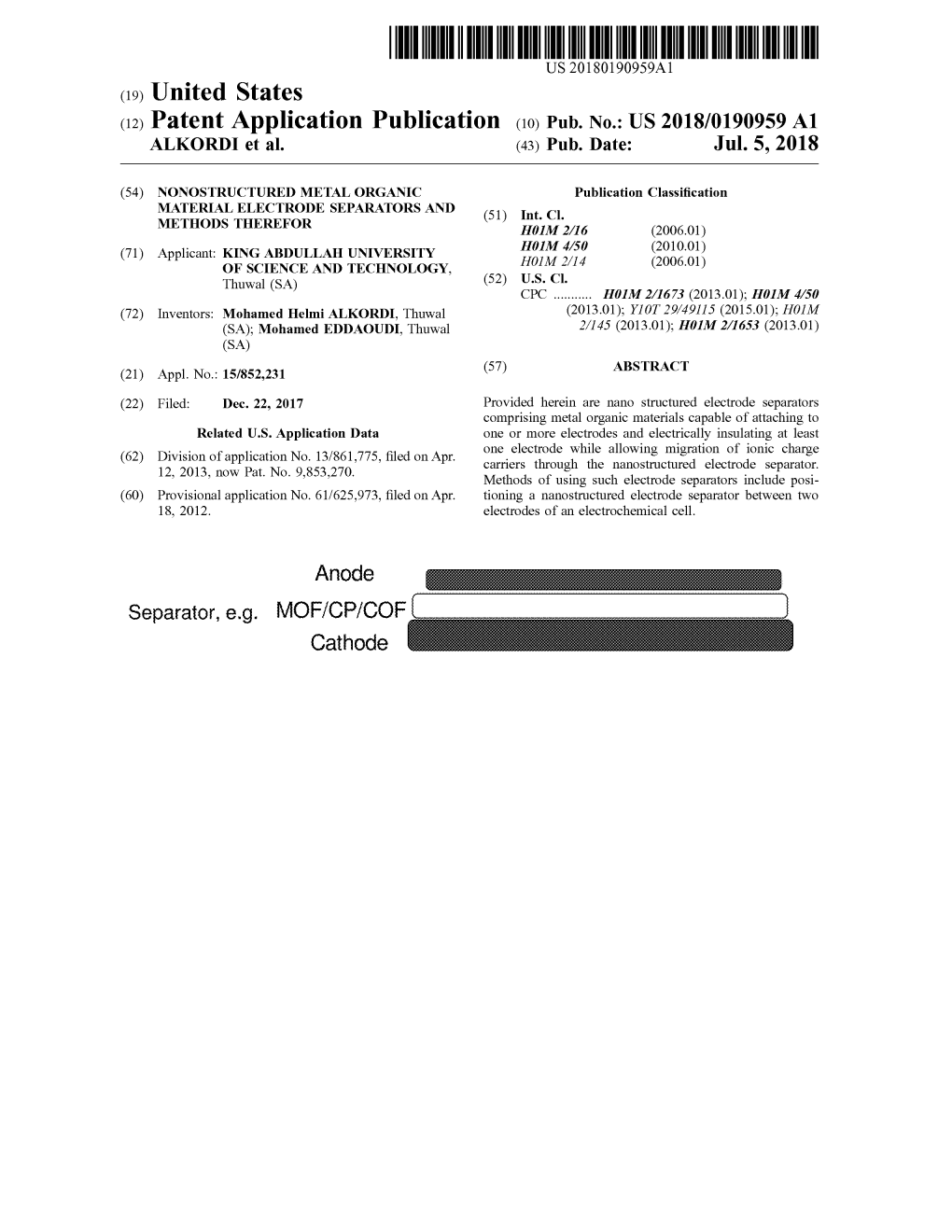
United States Oct. 24, 2013 Anode Cathode
US 20130280611A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0280611 A1 ALKORDI et al. (43) Pub. Date: Oct. 24, 2013 (54) ELECTRODE SEPARATOR Related US. Application Data (71) Applicant: King Abdullah University of Science (60) Provisional application No. 61/625,973, ?led on Apr. and Technology, (US) 18, 2012. Publication Classi?cation (72) Inventors: Mohamed Helmi ALKORDI, ThuWal (SA); Mohamed EDDAOUDI, ThuWal (51) Int. Cl. (SA) H01M 2/16 (2006.01) H01M 2/14 (2006.01) (73) Assignee: King Abdullah University of Science (52) US. Cl. and Technology, ThuWal (SA) CPC .......... .. H01M2/1673 (2013.01); H01M2/145 (2013.01) USPC ........... .. 429/224; 429/246; 29/623.5; 427/58 Appl. No.: 13/861,775 (21) (57) ABSTRACT A nanostructured separator for a battery or electrochemical (22) Filed: Apr. 12, 2013 cell can be a nanostructured separator. Anode Separator, 9.9. MOF/CP/COF , Cathode Patent Application Publication Oct. 24, 2013 Sheet 1 0f 13 US 2013/0280611 A1 Anode Separator, e.g. MOF/CP/COF _ Cathode Fig. 1 Patent Application Publication Oct. 24, 2013 Sheet 2 0f 13 US 2013/0280611 A1 Bridging group Polyvalentoore m Fig.2A Bridging group \ Polyvaient core — Fig.2B Bridging group \ Polyvalent core // Fig. 2C Patent Application Publication Oct. 24, 2013 Sheet 3 0f 13 US 2013/0280611 A1 .. .. Patent Application Publication Oct. 24, 2013 Sheet 4 0f 13 US 2013/0280611 A1 Fig. 4A Fig. 4B Patent Application Publication Oct. 24, 2013 Sheet 5 0f 13 US 2013/0280611 A1 Patent Application Publication Oct. 24, 2013 Sheet 6 0f 13 US 2013/0280611 A1 aa“Aa gmmmwmwgwmmwx wmun» w 28 25 39 35 A3 #5 28 Fig. -

I66 the CADMIUM STANDARD CELL. a STANDARD Cell with A
i66 G. A. HULETT. [VOL. XXIII. THE CADMIUM STANDARD CELL. BY GEORGE A. HULETT. A STANDARD cell with a low temperature coefficient was de- ^~*- vised by Mr. Edward Weston in 1891.1 By substituting cad mium sulphate and cadmium amalgam for the zinc sulphate and zinc of the Clark cell, it was found that the temperature coefficient of the cell was reduced 95 per cent., and when the solid cadmium sulphate was dispensed with and only a solution of cadmium sul phate used in the cell, the temperature coefficient was still less. With a solution saturated at o° C, Barnes and Lucas found2 that the E.M.F. of the cell was constant for all ordinary temperature changes. This latter combination is a valuable laboratory instru ment, but is not sufficiently reproducible or constant to serve as a standard of E.M.F. The Clark cell has a variation of E.M.F. of over a millivolt per degree, and for accurate work requires a very exact control of the temperature for a considerable time. The saturated cadmium cell has only one twentieth of the temperature coefficient of the Clark cell and many other desirable features, so that considerable work has been done in testing its reproducibility and constancy. A. Dearlove3 found that attention must be given to the cadmium amalgam and concluded that it was best to use a 12.5 per cent. amalgam, made by melting together one part of cadmium and seven parts of mercury. Since then considerable work has been done on cadmium amalgams. Kerp and Boettger4 concluded that the solid which separates from cadmium amalgams of over 5 per cent, was a compound represented by the formula Cd2Hgr However, from the work of Bijl5 and Puschin 6 it appears that between about 5 and 15 LU. -

An Examination of the Catalyst Layer Contribution to the Disparity Between the Nernst Potential and Open Circuit Potential in Proton Exchange Membrane Fuel Cells
catalysts Article An Examination of the Catalyst Layer Contribution to the Disparity between the Nernst Potential and Open Circuit Potential in Proton Exchange Membrane Fuel Cells Peter Mardle 1 , Isotta Cerri 2, Toshiyuki Suzuki 3 and Ahmad El-kharouf 1,* 1 School of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] 2 Toyota Motor Europe, Hoge Wei 33, 1930 Zaventem, Belgium; [email protected] 3 Toyota Motor Corporation, Toyota City 471-8571, Japan; [email protected] * Correspondence: [email protected]; Tel.: +44-121-414-5081 Abstract: The dependency of the Nernst potential in an operating proton exchange membrane fuel cell (PEMFC) on the temperature, inlet pressure and relative humidity (RH) is examined, highlighting the synergistic dependence of measured open circuit potential (OCP) on all three parameters. An alternative model of the Nernst equation is derived to more appropriately represent the PEMFC system where reactant concentration is instead considered as the activity. Ex situ gas diffusion electrode (GDE) measurements are used to examine the dependency of temperature, electrolyte concentration, catalyst surface area and composition on the measured OCP in the absence of H2 crossover. This is supported by single-cell OCP measurements, wherein RH was also investigated. This contribution provides clarity on the parameters that affect the practically measured OCP as well Citation: Mardle, P.; Cerri, I.; Suzuki, as highlighting further studies into the effects of catalyst particle surrounding environment on OCP T.; El-kharouf, A. An Examination of as a promising way of improving PEMFC performance in the low current density regime. -

Dissolved Oxygen Measurement
Dissolved Oxygen Measurement Introduction • Electrolyte - The electrolyte facilitates dissolved oxygen migration and provides an electrical path Dissolved oxygen is defined as the measure of water to complete the current loop. It also removes metal quality indicating free oxygen dissolved in water. The oxides (a by-product of the reaction) from the quantity of dissolved oxygen in water is typically electrodes so that their metal surfaces are clean to expressed in parts per million (ppm) or milligrams per react. Electrolyte must be periodically replenished liter (mg/l). Since oxygen is soluble in water, the to insure that the electrodes remain clean. amount of dissolved oxygen in water is in the state of dynamic equilibrium. The solubility of the dissolved The operational theory of a membrane sensor is that oxygen is proportional to the temperature and oxygen in the wastewater diffuses through the pressure of the water. membrane into the electrolyte. The concentration of gases always tends to equalize on both sides of the The most common application for dissolved oxygen membrane. When the concentration is not equal, gas measurement occurs in wastewater treatment. molecules migrate to the membrane side that has a Biochemical breakdown of sewage is achieved by lower concentration. When the membrane is bacterial attack in the presence of oxygen. This functioning, dissolved oxygen concentration in the process typically takes place in an aeration basin of a electrolyte in the measurement cell approximately wastewater treatment plant, and is accomplished by equals the dissolved oxygen concentration of the aerating or bubbling air (or pure oxygen) through the wastewater contacting the opposite side of the wastewater. -

A Convenient Standard Cell
Proceedings of the Iowa Academy of Science Volume 22 Annual Issue Article 23 1915 A Convenient Standard Cell Dieu Ung Huong State University of Iowa J. N. Pearce State University of Iowa Let us know how access to this document benefits ouy Copyright ©1915 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Huong, Dieu Ung and Pearce, J. N. (1915) "A Convenient Standard Cell," Proceedings of the Iowa Academy of Science, 22(1), 169-174. Available at: https://scholarworks.uni.edu/pias/vol22/iss1/23 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Huong and Pearce: A Convenient Standard Cell CONVENIENT STANDARD CELL 169 A CONVENIENT STANDARD CELL. DIED UNG HUONG AND J. N. PEARCE. Until receptly both the Clark and the Weston cells have served as standard sources of electromotive force. Both of these con sist ofl an amalgam of a metal as the anode covered by a satur ated solution of the sulphate of the metal and this in conjunc tion with mercury and mercurous sulphate which serves as the cathode. Clark cell: (Hg-Zn)-ZnS04-Hg2SO,-Hg. Weston cell: (Hg-Cd)-CdSO,-Hg2SO,-Hg. For various reasons the acceptance of the Clark cell as a standard has been discontinued. The Weston cell, chiefly on account of its approximately negligible temperature coefficient, is now the sole accepted standard of electromotive force. -

Water Quality Procedures
WAB Field SOP 2018 Revision Date: 8/22/2018 CHAPTER 3. WATER COLLECTION PROTOCOLS Overview A sonde is a collection of multiple sensors and probes housed into one casing and designed to give readings on multiple parameters simultaneously (often referred to as a Multi-Parameter Sonde). Sondes can vary in length, diameter, and housing construction. The most common four sensors on sondes are Temperature, pH, Dissolved Oxygen, and Specific Conductance (typically housed in a 2-inch diameter casing). Larger sondes (e.g., 3.5 – 4-inch diameter casing) can be equipped with additional, larger sensors and probes (e.g., Depth, Chlorophyll-a, and Turbidity). On some sondes (e.g., YSI models) the sensors and probes can easily be changed or replaced by the user. On other sondes (e.g., Hydrolab models), the sensors and probes cannot be easily changed without opening the sonde housing and electronics (either by a very experienced user or by sending the instrument back to the manufacturer’s service department). Figure 3-1. Example of a YSI 660 XL Sonde (right) and a 650 MDS Display Unit (left). Sensors and probes may utilize drastically contrasting technologies to measure a parameter. For example, one sonde may have a Dissolved Oxygen sensor that utilizes Water Collection-Overview Page | 3-1 WAB Field SOP 2018 Revision Date: 8/22/2018 the smaller Clark-cell technology (it has a membrane) with a stirrer; another sonde may use a similar Clark-cell technology and not have a stirrer; yet another sonde may use the larger Luminescence (LDO) technology (no membrane). In this case, the different sensor/probe technologies respectively offer pros and cons based on size the accuracy of the reading and size of the sonde casing required to house the sensor. -

Clark and Weston Standard Cells
CLARK AND WESTON STANDARD CELLS. By F. A. Wolff and C. E. Waters. INTRODUCTION. The important role now played by the standard cell in both tech- nical and scientific work, and the possibility of its adoption as a primary standard of electromotive force, have led in recent years to a considerable number of investigations concerning its reproduci- bility and constancy. The character of these investigations and the results obtained will, however, be better understood after a brief review of the previous work on the subject. The need of a definite and universal system of electrical units was early recognized. Owing to its preponderating importance in the earlier applications of electricity, the unit of resistance naturally received first attention. The committee on electrical standards appointed by the British Association in 1861 recommended the adoption of the C. G. S. electromagnetic system together with a practical system defined as decimal multiples and submultiples of the C. G. S. units. In addition, its labors led to the construction of concrete standards of resistance in the form of coils of platinum- 9 silver of special design adjusted to represent io C. G. S. units as determined by a series of absolute measurements. The definition of unit current and electromotive force in terms of the C. G. S. units long met every requirement, particularly as currents were generally measured by the aid of the tangent galvanometer, while electromotive forces were generally measured in terms of the electromotive force of the Daniell cell. In 1872 Latimer Clark called to the attention of the British Asso- ciation committee the superiority of the cell which now bears his 2 Bulletin of the Bureau of Standards. -

Miniaturized Planar Room Temperature Ionic Liquid Electrochemical Gas
Sensors and Actuators B 255 (2018) 638–646 Contents lists available at ScienceDirect Sensors and Actuators B: Chemical jo urnal homepage: www.elsevier.com/locate/snb Miniaturized planar room temperature ionic liquid electrochemical gas sensor for rapid multiple gas pollutants monitoring a,∗ a b b,∗ a,∗ Hao Wan , Heyu Yin , Lu Lin , Xiangqun Zeng , Andrew J. Mason a Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48824, USA b Department of Chemistry, Oakland University, Rochester, MI, 48309, USA a r a t i b s c t l e i n f o r a c t Article history: The growing impact of airborne pollutants and explosive gases on human health and occupational safety Received 28 April 2017 has escalated the demand of sensors to monitor hazardous gases. This paper presents a new miniaturized Received in revised form 4 August 2017 planar electrochemical gas sensor for rapid measurement of multiple gaseous hazards. The gas sensor Accepted 12 August 2017 features a porous polytetrafluoroethylene substrate that enables fast gas diffusion and room tempera- Available online 18 August 2017 ture ionic liquid as the electrolyte. Metal sputtering was utilized for platinum electrodes fabrication to enhance adhesion between the electrodes and the substrate. Together with carefully selected electro- Keywords: chemical methods, the miniaturized gas sensor is capable of measuring multiple gases including oxygen, Electrochemical gas sensor methane, ozone and sulfur dioxide that are important to human health and safety. Compared to its Miniaturized sensor manually-assembled Clark-cell predecessor, this sensor provides better sensitivity, linearity and repeata- Planar platinum electrode Room temperature ionic liquid bility, as validated for oxygen monitoring.