5 Koster's Curse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Koster's Curse (448) Relates To: Weeds
Pacific Pests, Pathogens & Weeds - Fact Sheets https://apps.lucidcentral.org/ppp/ Koster's curse (448) Relates to: Weeds Photo 1. Mass of seedlings of Koster's curse, Clidemia Photo 2. Leaves, Koster's curse, Clidemia hirta. Note, hirta. the distinctive veins patterns. Photo 3. Flowers, Koster's curse, Clidemia hirta. Note, Photo 4. Flowers and developing fruits, Koster's curse, the five petals. Clidemia hirta. Photo 6. Flowers and fruits, Koster's curse, Clidemia Photo 5. Flowers and fruits, Koster's curse, Clidemia hirta. Note, the claw-like stamens, and hairs on the hirta. fruits. Photo 7. Fruits, Koster's curse, Clidemia hirta, showing hairs and bristles on fruits and leaf stalks. Common Name Koster's curse; it is also known as soapbush. Scientific Name Clidemia hirta. It was known previously as Clidemia elegans, Melastoma elegans. It is a member of the Melastomataceae. Distribution Widespread. Asia, East Africa, South and Southeast Asia, North, South and Central America, the Caribbean, Oceania. It is recorded from Australia, American Samoa, Fiji, Guam, Palau, Papua New Guinea, Samoa, Solomon Islands, Tonga, Vanuatu, and Wallis and Futuna. Koster's curse is native to much of tropical America. Invasiveness & Habitat An extremely important invasive weed, and especially a threat to Pacific islands. Koster's curse forms dense thickets that smother plantations, pastures and native vegetation, but it is also found in open grasslands, roadsides, open woodlands, banks of streams and rivers, forest margins and rainforests (Photo 1). The weed invades both disturbed and undisturbed areas, but it is especially problematic after storms, feral pig damage, landslides and fire. -

Restricted Invasive Plants of Queensland
Restricted invasive plants Restricted invasive plants of Queensland Restricted invasive plants of Queensland Hudson pear (Cylindropuntia rosea syn. Cylindropuntia pallida) Fireweed (Senecio madagascariensis) Mother-of-millions (Kalanchoe delagoense) Bunny ears (Opuntia microdasys) The new Biosecurity Act The Biosecurity Act 2014 protects Queensland’s economy, Species not listed as restricted may be listed as prohibited biodiversity and people’s lifestyles from the threats posed under the Act or may be listed by a local government level by invasive pests and diseases under local laws. Under the Act, certain species of invasive plants are listed Australian Government legislation administered by the as ‘restricted’ biosecurity matter. Australian Department of Agriculture also applies to the import of all plants into Australia. What is restricted matter? • Mexican bean tree (Cecropia pachystachya, C. palmata and C. peltata) Restricted matter is listed in the Act and includes a range • Mexican feather grass (Nassella tenuissima) of invasive plants that are present in Queensland. These invasive plants are having significant adverse impacts • miconia (M. calvescens, M. cionotricha, M. nervosa in Queensland and it is desirable to manage them and and M. racemosa) prevent their spread, thereby protecting un-infested • mikania vine (Mikania micrantha) parts of the State. • mimosa pigra (Mimosa pigra) The Act requires everyone to take all reasonable and practical measures to minimise the biosecurity risks • bunny ears (Opuntia microdasys) associated with invasive plants and animals under • riverina prickly pear (Opunita elata) their control. This is called a general biosecurity obligation (GBO). • water mimosa (Neptunia oleracea and N. plena). The specific restriction requirements also apply to a Restricted invasive plants that are person when dealing with restricted invasive matter. -

Morphoanatomical Study of Clidemia Hirta (L.) D. Don. Estudo Morfoanatômico De Clidemia Hirta (L.) D
Research, Society and Development, v. 10, n. 7, e1310716159, 2021 (CC BY 4.0) | ISSN 2525-3409 | DOI: http://dx.doi.org/10.33448/rsd-v10i7.16159 Morphoanatomical study of Clidemia hirta (L.) D. Don. Estudo morfoanatômico de Clidemia hirta (L.) D. Don. Estudio morfoanatómico de Clidemia hirta (L.) D. Don. Received: 05/16/2021 | Reviewed: 05/25/2021 | Accept: 05/26/2021 | Published: 06/11/2021 Tatiane Mendonça da Silva ORCID: https://orcid.org/0000-0002-3553-901X Universidade Federal de Goiás, Brazil E-mail: [email protected] Heleno Dias Ferreira ORCID: https://orcid.org/0000-0001-7763-734X Universidade Federal de Goiás, Brazil E-mail: [email protected] José Realino de Paula ORCID: https://orcid.org/0000-0002-4424-7692 Universidade Federal de Goiás, Brazil E-mail: [email protected] Tatiana de Sousa Fiuza ORCID: https://orcid.org/0000-0003-0135-177X Universidade Federal de Goiás, Brazil E-mail: [email protected] Abstract The aims of this study were: to carry out the morphological study of the Clidemia hirta (L.) D. Don, the anatomical study of the leaves and young stem and the phytochemical screening of the powder of the leaves. The leaves were collected monthly at Bosque Auguste de Saint-Hilaire, Conservation Unit on Campus II of the Federal University of Goiás (UFG), Goiânia, Goiás, for 12 months. The specie was identified and a voucher specime deposited in the Herbarium of UFG (66 872- UFG). Morphoanatomical analysis was performed according to conventional techniques. It was verified at phytochemical screening, the presence de anthraquinone heterosides, starch, alkaloids, flavonoid and saponins. -

Review and Status of Biological Control of Clidemia in Hawaici
REVIEW AND STATUS OF BIOLOGICAL CONTROL OF CLIDEMIA IN HAWAICI Larry M. Nakahara, Robert Me Burkhart, and George Ye Funasaki ABSTRACT Efforts to control clidemia (Clidemia hirta) in Hawai'i with phytophagous insects began in 1952. Several attempts have been made since then to introduce potential biological control agents to reduce the spread of this weed into forest areas, and other control measures have been tried by various groups and agencies. It was not until recently, however, through the enactment of significant legislation and subsequent funding, that explorations and studies were conducted specifically on clidemia insects in Trinidad, West Indies. Fourteen species of insects were evaluated, including Carposina bullata, Mompha bithalama, a midge or cecidomyiid, two Eurytoma species, Piesmopoda sp., and Compsolechia seductella, which feed on the flowers and fruits; Lius poseidon, Antiblemma acclinalis, Druentia sp., prob. inscita, Ategumia matutinalis (formerly Blepharomastix ebulealis but originally thought to be Sylepte matutinalis), Penestes n. sp., and a leaf beetle or chrysomelid, which feed on the leaves; and a long-horned cerambycid beetle, which bores into the stem. Studies indicated that several species were sufficiently host specific to warrant introduction into Hawai'i for the biological control of clidemia. INTRODUCTION In Hawai'i, clidemia or Koster's curse (Clidemia hirta) (Melastomataceae), a fast-growing, hea -seeding, tropical American shrub, has colonized forest clearings, trailsi7 es, and bum sites and intruded into the understories of forests that were formerly free of introduced, or alien, weeds (Wester and Wood 1977). The species has spread rapidly throughout the State and re resents a serious threat to our native forests. -

9:00 Am PLACE
CARTY S. CHANG INTERIM CHAIRPERSON DAVID Y. IGE BOARD OF LAND AND NATURAL RESOURCES GOVERNOR OF HAWAII COMMISSION ON WATER RESOURCE MANAGEMENT KEKOA KALUHIWA FIRST DEPUTY W. ROY HARDY ACTING DEPUTY DIRECTOR – WATER AQUATIC RESOURCES BOATING AND OCEAN RECREATION BUREAU OF CONVEYANCES COMMISSION ON WATER RESOURCE MANAGEMENT STATE OF HAWAII CONSERVATION AND COASTAL LANDS CONSERVATION AND RESOURCES ENFORCEMENT DEPARTMENT OF LAND AND NATURAL RESOURCES ENGINEERING FORESTRY AND WILDLIFE HISTORIC PRESERVATION POST OFFICE BOX 621 KAHOOLAWE ISLAND RESERVE COMMISSION LAND HONOLULU, HAWAII 96809 STATE PARKS NATURAL AREA RESERVES SYSTEM COMMISSION MEETING DATE: April 27, 2015 TIME: 9:00 a.m. PLACE: Department of Land and Natural Resources Boardroom, Kalanimoku Building, 1151 Punchbowl Street, Room 132, Honolulu. AGENDA ITEM 1. Call to order, introductions, move-ups. ITEM 2. Approval of the Minutes of the June 9, 2014 N atural Area Reserves System Commission Meeting. ITEM 3. Natural Area Partnership Program (NAPP). ITEM 3.a. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $663,600 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Kapunakea Preserve Long Range Management Plan, TMK 4-4-7:01, 4-4-7:03, Lahaina, Maui. ITEM 3.b. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $470,802 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Pelekunu Long Range Management Plan, TMK 5-4- 3:32, 5-9-6:11, Molokai. -

Invasive Plants: Changing the Landscape of America
Utah State University DigitalCommons@USU All U.S. Government Documents (Utah Regional U.S. Government Documents (Utah Regional Depository) Depository) 1998 Invasive Plants: Changing the Landscape of America Federal Interagency Committee for the Management of Noxious and Exotic Weeds Randy G. Westbrooks Follow this and additional works at: https://digitalcommons.usu.edu/govdocs Part of the Environmental Indicators and Impact Assessment Commons Recommended Citation Federal Interagency Committee for the Management of Noxious and Exotic Weeds and Westbrooks, Randy G., "Invasive Plants: Changing the Landscape of America" (1998). All U.S. Government Documents (Utah Regional Depository). Paper 490. https://digitalcommons.usu.edu/govdocs/490 This Report is brought to you for free and open access by the U.S. Government Documents (Utah Regional Depository) at DigitalCommons@USU. It has been accepted for inclusion in All U.S. Government Documents (Utah Regional Depository) by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. DISCLAIMER This document COlltains . tone-on-tone or color graphs, charts and/or pictuTes Wllicll l1ave been reproduced in black and white. disk 1 cvrtone. FC'defn I In tcra ~'i('n('v Comlnitt(>C' for the { .' Mlln;H',cment of (lnd Exotic t 1 I), PROTECTED UNDER INTERNATIONAL COPYRIGHT ALL RIGHTS RESERVED. NATIONAL TECHNICAL INFORMATION SERVICE U.S. DEPARTMENT OF COMMERCE Cataloging-in-Publication Data Westbrooks, Randy G., 1953- Invasive plants: changing the landscape of America: fact book! [senior author, Randy Westbrooks]. -- Washington, D.C.: Federal Interagency Committee for the Management of Noxious and Exotic Weeds, 1998. [vi], 107 p.: col. Ill.; 28 cm. -

Clidemia Hirta (L.) D
Crop Protection Compendium - Clidemia hirta (L.) D. Don Pierre Binggeli 2005 NAMES AND TAXONOMY Preferred scientific name Clidemia hirta (L.) D. Don Taxonomic position Other scientific names Domain: Eukaryota Melastoma hirta sensu Miller Kingdom: Viridiplantae Melastoma hirtum L. Phylum: Spermatophyta Clidemia crenata DC. Subphylum: Angiospermae Melastoma elegans Aublet Class: Dicotyledonae Clidemia elegans (Aublet) D. Don Order: Myrtales Melastoma hirta L. Family: Melastomataceae BAYER code CXAHI (Clidemia hirta) Common names English: Belau: Madagascar: Koster's curse kui manzana soap bush kúi mazambôdy curse Fiji: Martinique: Spanish: Koster's curse bonbon bleu camasey bona na bulamakau herbe à cré cré camasey peludo kaurasinga Portugal: nigua kauresinga caiuia sietecueros mara na bulumakau pixirica French: mbona na mbulamakau Samoa: canot-macaque ndraunisinga la'au lau mamoe herbe-côtelletes roinisinga Singapore: herbecrécré vuti hairy Clidemia Mélastome élégant Haiti: Mélastome poilu guéri vite Notes on taxonomy and nomenclature Varieties of C. hirta have been described. Var. hirta and var. elegans were introduced to Hawaii and the Seychelles, respectively. Binggeli 2005 Crop Protection Compendium - Clidemia hirta (L.) D. Don 1 HOST RANGE Major hosts Cocos nucifera (coconut), Hevea brasiliensis (rubber), Theobroma cacao (cocoa) Minor hosts pastures HABITAT Most tropical island forest areas appear to be susceptible to C. hirta invasion regardless of their floristic composition, as long as some form of disturbance affects them. In Hawaii all new instances of C. hirta occur in disturbed areas such as roadsides and landslides and following disturbance by windstorm, pigs, landslides and fire. In the East Usambaras (Tanzania) the shrub is found not only along roadsides but also in many parts of the undisturbed montane forest (Binggeli, 2003). -

Moths and Butterflies
LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004MOTHS LJL©2004 LJL©2004AND BUTTERFLIES LJL©2004 LJL©2004 (LEPIDOPTERA) LJL©2004 LJL©2004 LJL©2004 FROM LJL©2004 BAHÍA LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004HONDA LJL©2004 LJL©2004 AND CANALES LJL©2004 LJL©2004 DE TIERRA LJL©2004 ISLANDLJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004(VERAGUAS, LJL©2004 LJL©2004 PANAMA LJL©2004) LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 -

Clidemia Hirta Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Clidemia hirta Clidemia hirta System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Magnoliopsida Myrtales Melastomataceae Common name kauresinga (English), kaurasiga (English), mbona na mbulamakau (English), ndraunisinga (English), roinisinga (English), vuti (English), soap bush (English), Hirten-Schwarzmundgewaechs (German), Koster's curse (English), faux vatouk (English), clidemia, kúi Synonym Melastoma hirtum , L. Similar species Summary The invasive shrub Clidemia hirta is a problem in tropical forest understories in its introduced range, where it invades gaps in the forest, preventing native plant species from regenerating. The spread of Clidemia hirta has been linked to soil disturbances, particularly that caused by the wild pig, another invasive species. It has proven to negatively effect native ecosystems and is difficult to control in the Hawaiian archipelago. It is feared it will have a similar effect in other regions where it has been introduced such as in various Indian Ocean Islands (Seychelles), the Malaysian Peninsula and parts of Micronesia (Palau). view this species on IUCN Red List Species Description Koster’s curse is a coarse perennial shrub up to 2m tall. The stems are covered with red bristles that lighten with age. The leaves are opposite, simple and petiolate. The ovate-to-oblong leaf blades are hairy with crenate margins. The surfaces appear pleated. Five major veins originate at the base of the leaf and extend to the apex. The inflorescence is a panicle that can be terminal or axillary. The calyx has five hairy linear lobes atop a long urceolate hypanthium. The corolla consists of five small white petals. The fruit is a hairy ovoid many seeded bluish-black berry (Weedy Plants of the US Undated). -

Two Species of the Mompha Trithalama-Complex As Possible Biological Control of Koster's Curse and Velvet Tree
Two species of the Mompha trithalama-complex as possible biological control of Koster’s curse and Velvet tree Sjaak Koster, Manuel Alfaro Alpízar, Francisco R. Badenes-Pérez & Kenji Nishida Mompha spec. A Mompha trithalama Mompha spec. B Mompha trithalama Mompha spec. A Mompha spec. B Mompha trithalama Mompha spec. A Clidemia hirta (L.) D.Don (Melastomataceae) Koster’s curse or soap bush Photo: www.cybertruffle.org.uk/vinales/esp/clidemia_hirta Clidemia hirta (L.) D.Don (Melastomataceae) Koster’s curse or soap bush Photo courtesy Konrad Englberger, © Secretariat of the Pacific Community Geographical distribution of Clidemia hirta in areas where it is native (circles) and introduced (squaires) (DeWalt, 2003) Damage to the native flora Photo courtesy Konrad Englberger, © Secretariat of the Pacific Community Damage to the native flora Clidemia hirta on Babeldaob, Palau Photo by Jim Space, PIER Attempts for biological control so far The fungus: Colletotrichum gloesporioides Penz. (Phyllachorales: Phyllachoraceae) The thrips: Liothrips urichi Karny (Thysanoptera: Phlaeothripidae) The beetle: Lius poseidon Napp (Coleoptera: Buprestidae) The moths: Antiblemma acclinalis Hübner (Noctuidae) Carposina bullata Meyrick (Carposinidae) Mompha trithalama Meyrick (Momphidae) Miconia calvescens Photo’s by The Nature Conservancy Archive, The Nature Conservancy Photo’s Wikipedia and http://pbin.nbii.gov/reportapest/maui/pestlist/miccal.htm Mark that the larva made when it got into the berry Photo Manuel Alfaro Alpízar Larva inside the berry Photo Manuel Alfaro Alpízar Fruits of Miconia calvescens with the larva of Mompha spec. A Photo Manuel Alfaro Alpízar Different instars of larvae of Mompha spec. A Photo Manuel Alfaro Alpízar Construction of cocoon Photo Manuel Alfaro Alpízar Thank you for your attention Abstract of my presentation held on the XV European Congress of Lepidopterology at Berlin from 8 – 12 september 2007. -
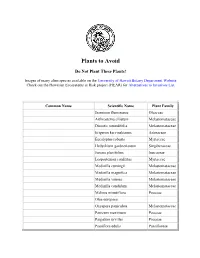
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

The Invasive Tropical Shrub Clidemia Hirta (Melastomataceae) in Its Native and Introduced Ranges: Tests of Hypotheses of Invasio
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 The invasive tropical shrub Clidemia hirta (Melastomataceae) in its native and introduced ranges: tests of hypotheses of invasion Saara Jennie DeWalt Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation DeWalt, Saara Jennie, "The invasive tropical shrub Clidemia hirta (Melastomataceae) in its native and introduced ranges: tests of hypotheses of invasion" (2003). LSU Doctoral Dissertations. 2030. https://digitalcommons.lsu.edu/gradschool_dissertations/2030 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. THE INVASIVE TROPICAL SHRUB CLIDEMIA HIRTA (MELASTOMATACEAE) IN ITS NATIVE AND INTRODUCED RANGES: TESTS OF HYPOTHESES OF INVASION A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Saara Jennie DeWalt A.B., Brown University, 1994 May 2003 Acknowledgements During the course of my dissertation research, I have had the pleasure of working and studying with many people in several parts of the world. Their generous support, suggestions, and help made this work possible as well as enjoyable. At Louisiana State University (LSU), Julie Denslow was a wonderful advisor throughout my time as a graduate student. She helped me make the contacts to start this project, critically reviewed numerous proposals and papers, and encouraged me when my spirits flagged.