Injections: Drugs AD Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Thrombin and Thromboxane A2 in Reocclusion Following Coronary
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 7585-7589, October 1989 Medical Sciences Role of thrombin and thromboxane A2 in reocclusion following coronary thrombolysis with tissue-type plasminogen activator (thrombolytic therapy/coronary thrombosis/platelet activation/reperfusion) DESMOND J. FITZGERALD*I* AND GARRET A. FITZGERALD* Divisions of *Clinical Pharmacology and tCardiology, Vanderbilt University, Nashville, TN 37232 Communicated by Philip Needleman, June 28, 1989 (receivedfor review April 12, 1989) ABSTRACT Reocclusion of the coronary artery occurs against the prothrombinase formed on the platelet surface after thrombolytic therapy of acute myocardlal infarction (13) and the neutralization ofheparin by platelet factor 4 (14) despite routine use of the anticoagulant heparin. However, and thrombospondin (15), proteins released by activated heparin is inhibited by platelet activation, which is greatly platelets. enhanced in this setting. Consequently, it is unclear whether To address the role of thrombin during coronary throm- thrombin induces acute reocclusion. To address this possibility, bolysis, we have examined the effect of a specific thrombin we examined the effect of argatroban {MCI9038, (2R,4R)- inhibitor, argatroban {MCI9038, (2R,4R)-4-methyl-1-[N-(3- 4-methyl-l-[Na-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfo- methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl]-2- nyl)-L-arginyl]-2-piperidinecarboxylic acid}, a specific throm- piperidinecarboxylic acid} on the response to tissue plasmin- bin inhibitor, on the response to tissue-type plasminogen ogen activator (t-PA) in a closed-chest canine model of activator in a dosed-chest canine model of coronary thrombo- coronary thrombosis. MCI9038, an argimine derivative which sis. MCI9038 prolonged the thrombin time and shortened the binds to a hydrophobic pocket close to the active site of time to reperfusion (28 + 2 min vs. -

Stroke Prevention in Chronic Kidney Disease Disclosures
5/18/2020 Controversies: Stroke Prevention in Chronic Kidney Disease Wei Ling Lau, MD FASN FAHA FACP Assistant Professor, Nephrology University of California, Irvine Visiting Fellow at OptumLabsCOPY Disclosures • Prior or current research funding from NIH, AHA, Sanofi, ZS Pharma, and Hub Therapeutics. • Associate Medical Director for home peritoneal dialysis at Fresenius University Dialysis Center of Orange. • Has beenNOT on Fresenius medical advisory board for Velphoro. • No conflicts of interest relevant to the current talk. Controversies: Stroke prevention in CKD Wei Ling Lau, MD DO 1 5/18/2020 Stroke Prevention in CKD • Blood pressure targets • Antiplatelet agents • Statins • Anticoagulation Controversies: Stroke prevention in CKD Wei Ling Lau, MD COPY BP TARGETS Data is limited, as patients with CKD were historically excluded from clinical trials NOT Whelton 2017 ACC/AHA hypertension guidelines [Hypertension 2018] Controversies: Stroke prevention in CKD Wei Ling Lau, MD DO 2 5/18/2020 Systolic Blood Pressure Intervention Trial SPRINT: BP lowering to <120 vs <140 mmHg significantly lowered rate of CVD composite primary outcome; no clear effect on stroke Controversies: Stroke prevention in CKD The SPRINT Research Group. N Engl J Med 2015 p2103 Wei Ling Lau, MD COPY SPRINT subgroup analysis: CKD • Patients with CKD stage 3‐4 (eGFR of 20 to <60) comprised 28% of the SPRINT study population • Intensive BP management seemed to provide the same benefits for reduction in the CVD composite primary outcomeNOT – but did not impact stroke Controversies: Stroke prevention in CKD Cheung 2017 J Am Soc Nephrol p2812 Wei Ling Lau, MD DO 3 5/18/2020 The hazard of incident stroke associated with systolic BP (SBP) and chronic kidney disease (CKD)BP using and an unadjusted stroke model risk: that contained J‐shaped dummy variables association for CKD and BP groups (A) and a fully adjusted model that contained dummy variables for CKD and BP grou.. -

GTH 2021 State of the Art—Cardiac Surgery: the Perioperative Management of Heparin-Induced Thrombocytopenia in Cardiac Surgery
Review Article 59 GTH 2021 State of the Art—Cardiac Surgery: The Perioperative Management of Heparin-Induced Thrombocytopenia in Cardiac Surgery Laura Ranta1 Emmanuelle Scala1 1 Department of Anesthesiology, Cardiothoracic and Vascular Address for correspondence Emmanuelle Scala, MD, Centre Anesthesia, Lausanne University Hospital (CHUV), Lausanne, Hospitalier Universitaire Vaudois, Rue du Bugnon 46, BH 05/300, 1011 Switzerland Lausanne, Suisse, Switzerland (e-mail: [email protected]). Hämostaseologie 2021;41:59–62. Abstract Heparin-induced thrombocytopenia (HIT) is a severe, immune-mediated, adverse drug Keywords reaction that paradoxically induces a prothrombotic state. Particularly in the setting of ► Heparin-induced cardiac surgery, where full anticoagulation is required during cardiopulmonary bypass, thrombocytopenia the management of HIT can be highly challenging, and requires a multidisciplinary ► cardiac surgery approach. In this short review, the different perioperative strategies to run cardiopul- ► state of the art monary bypass will be summarized. Introduction genicity of the antibodies and is diagnostic for HIT. The administration of heparin to a patient with circulating Heparin-induced thrombocytopenia (HIT) is a severe, im- pathogenic HITabs puts the patient at immediate risk of mune-mediated, adverse drug reaction that paradoxically severe thrombotic complications. induces a prothrombotic state.1,2 Particularly in the setting The time course of HIT can be divided into four distinct of cardiac surgery, where full anticoagulation is required phases.6 Acute HIT is characterized by thrombocytopenia during cardiopulmonary bypass (CPB), the management of and/or thrombosis, the presence of HITabs, and confirma- HIT can be highly challenging, and requires a multidisciplin- tion of their platelet activating capacity by a functional ary approach. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Myelodysplastic Syndromes
MYELODYSPLASTIC SYNDROMES TREATMENT REGIMENS (Part 1 of 3) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. First-Line Treatment1 Note: All recommendations are Category 2A unless otherwise indicated. Relatively Lower-Risk Patientsa,b Symptomatic Anemia With del(5q) ± One Other Cytogenetic Abnormality (except those involving chromosome 7) REGIMEN DOSING Lenalidomide2-5,c Days 1–21: Lenalidomide 10mg orally once daily. Repeat cycle every 28 days (or 28 days monthly). Assess response 2–4 months after initiation of treatment. -

OMONTYS® Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- These highlights do not include all the information needed to use Dosage Form Strengths OMONTYS® safely and effectively. See full prescribing information for OMONTYS. Single use vials 2 mg/0.5 mL, 3 mg/0.5 mL, (preservative-free) 4 mg/0.5 mL, 5 mg/0.5 mL, and OMONTYS® (peginesatide) Injection, 6 mg/0.5 mL for intravenous or subcutaneous use Single use pre-filled syringes 1 mg/0.5 mL, 2 mg/0.5 mL, Initial U.S. Approval: 2012 (preservative-free) 3 mg/0.5 mL, 4 mg/0.5 mL, 5 mg/0.5 mL, and 6 mg/0.5 mL WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL Multiple use vials 10 mg/mL and 20 mg/2 mL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, (with preservative) THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE -------------------------------CONTRAINDICATIONS------------------------------ See full prescribing information for complete boxed warning. Uncontrolled hypertension (4). Chronic Kidney Disease: In controlled trials, patients experienced greater risks for -----------------------WARNINGS AND PRECAUTIONS------------------------ death, serious adverse cardiovascular reactions, and stroke Increased Mortality, Myocardial Infarction, Stroke, and when administered erythropoiesis-stimulating agents (ESAs) Thromboembolism: Using ESAs to target a hemoglobin level of to target a hemoglobin level of greater than 11 g/dL (5.1). greater than 11 g/dL increases the risk of serious adverse No trial has identified a hemoglobin target level, ESA dose, or cardiovascular reactions and has not been shown to provide dosing strategy that does not increase these risks (5.1). additional benefits (5.1). -

Statistical Analysis Plan – Part 1
NCT03251482 Janssen Research & Development Statistical Analysis Plan – Part 1 A Randomized, Double-blind, Double-dummy, Multicenter, Adaptive Design, Dose Escalation (Part 1) and Dose-Response (Part 2) Study to Evaluate the Safety and Efficacy of Intravenous JNJ-64179375 Versus Oral Apixaban in Subjects Undergoing Elective Total Knee Replacement Surgery Protocol 64179375THR2001; Phase 2 JNJ-64179375 Status: Approved Date: 18 October 2017 Prepared by: Janssen Research & Development, LLC Document No.: EDMS-ERI-148215770 Compliance: The study described in this report was performed according to the principles of Good Clinical Practice (GCP). Confidentiality Statement The information in this document contains trade secrets and commercial information that are privileged or confidential and may not be disclosed unless such disclosure is required by applicable law or regulations. In any event, persons to whom the information is disclosed must be informed that the information is privileged or confidential and may not be further disclosed by them. These restrictions on disclosure will apply equally to all future information supplied to you that is indicated as privileged or confidential. 1 Approved, Date: 18 October 2017 JNJ-64179375 NCT03251482 Statistical Analysis Plan - Part 1 64179375THR2001 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................... 2 LIST OF IN-TEXT TABLES AND FIGURES ............................................................................................... -

Interactions with COUMADIN
Interactions with COUMADIN INDICATIONS • have kidney problems • take other medicines that increase your risk of COUMADIN® (warfarin sodium) is a prescription medicine used bleeding, including: to treat blood clots and to lower the chance of blood clots – a medicine that contains heparin forming in your body. Blood clots can cause a stroke, heart – other medicines to prevent or treat blood clots attack, or other serious conditions if they form in the legs or – non-steroidal anti-inflammatory drugs (NSAIDs) lungs. COUMADIN may have been prescribed to help you: • take warfarin sodium for a long time. Warfarin sodium is • Reduce your risk of forming blood clots if you have had a the active ingredient in COUMADIN. heart-valve replacement or if you have an irregular, rapid Call your doctor or seek immediate medical care heartbeat, called atrial fibrillation if you have any of the following signs or symptoms • Lower the risk of death if you’ve had a heart attack, as of bleeding: well as lowering your risk of having another heart attack, • pain, swelling, or discomfort stroke, and having blood clots move to another part of • headaches, dizziness, or weakness your body • unusual bruising (bruises that develop without known It is not known if COUMADIN is safe and effective in children. cause or grow in size) • nosebleeds or bleeding gums IMPORTANT SAFETY INFORMATION • bleeding from cuts takes a long time to stop • menstrual bleeding or vaginal bleeding that is heavier COUMADIN® (warfarin sodium) can cause bleeding than normal which can be serious -

Anticoagulation Protocol
ANTICOAGULATION PROTOCOL Federal Bureau of Prisons Clinical Guidance MARCH 2018 This Anticoagulation Protocol is made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant this guidance for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient specific. Consult the BOP Clinical Guidance Web page to determine the date of the most recent update to this document: http://www.bop.gov/resources/health_care_mngmt.jsp Federal Bureau of Prisons Anticoagulation Protocol Clinical Guidance March 2018 WHAT’S NEW IN THIS DOCUMENT? The following changes have been made to the BOP Anticoagulation Protocol since it was last issued in April 2013: • A new table has been added to Section 4, Heparin Products. See Table 2. Dosing of LMWHs for Treatment and Prevention. • In Section 5, Warfarin, the discussion has been expanded to include lifestyle factors and health conditions affecting warfarin therapy. See Interactions with Food, Drugs, Lifestyle, and Health Conditions. • A new Section 6 on Novel Oral Anticoagulants (NOACs) has been added. • Information on Treatment of Deep Venous Thrombosis (DVT) and Pulmonary Embolism (PE) has been updated in Section 7. • The Inmate Fact Sheet on Warfarin is now available in Spanish. See Appendix 9B. • The CHA2DS2-VASc score has replaced the CHADS2 score for predicting thromboembolic stroke risk in non-valvular atrial fibrillation. See Appendix 10, Table B. i Federal Bureau of Prisons Anticoagulation Protocol Clinical Guidance March 2018 TABLE OF CONTENTS 1. -

213026Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 213026Orig1s000 OTHER REVIEW(S) IMMUNOGENICITY ASSESSMENT Application Type NDA Application Number 213026 Submit Date 01/10/2020 Received Date 01/10/2020 Division/Office CDER/OND/ON/DNI Review Completion Date 01/10/2021 Product Name Casimersen Proposed Proprie tary AMONDYS 45 Name Error! Bookmark not defined. Pharmacologic Class PMO exon Skipping Applicant Sarepta Therapeutics, Inc. (b) (4) Applicant Proposed Duchenne muscular dystrophy (DMD) in Indication(s) patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. Immunogenicity Assessors Primary Assessor(s) Seth Thacker PhD Secondary Assessor (s) Daniela Verthelyi PhD MD Assessor Recommendation: The sponsor has submitted data for anti-dystrophin antibodies in the casimersen trials. These data were generated using assays that were developed for assessing anti-dystrophin antibodies in patients treated with eteplirsen and golodirsen and have already been deemed fit for use. The sponsor submitted anti-dystrophin ADA data for Study 4045-101, which had 12 patients enrolled No positive samples were found. The FPR for these assays in Study 4045-101 were 1.3%(IgG), 7.9% (IgE), and 39% (IgM) as calculated by the assessor. The sponsors has not submitted an assay for the detection of Casimersen-specific ADAs or provided a plan on how they assess the risk associated with the generation of novel epitopes in the dystrophin formed by exon 45 skipping. PMRs will be issued to the sponsor to develop and validate the assays and to assess the patients in study 4045-101 and 4045-301 for Abs to the product and to the peptide generated through the exon skipping strategy. -
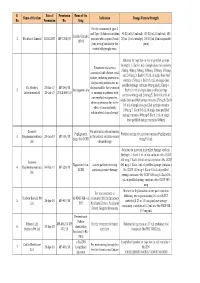
Final List of R-DNA Based Drugs Approved in the Country.Xlsx
S. Date of Permission Name of the Name of the firm Indication Dosage Form & Strength No. Permission No. Drug For the treatment of type -I and Type -II diabetes mellitus 40 IU/ml (10 ml vial), 100 IU/ml (10 ml vial), 100 Insulin Glargine 1 Wockhardt Limited 22.02.2007 MF-7206/07 patients who required basal IU/ml (3 ml cartridge), 100 IU/ml (3 ml disposable 100 IU (long acting) insulin for the pens) control of hyperglycemia. Solution for injection in vial or prefilled syringe Strength: 1. Each 1 mL of single dose vial contains Treatment of anaemia 25mcg, 40mcg, 60mcg, 100mcg, 200mcg, 300mcg associated with chronic renal and 500mcg 2. Each 0.75 mL of single dose vial failure, including patients on contains 150mcg 3. Each 0.3 mL of single dose dialysis and patients not on prefilled syringe contains 60mcg and 150mcg 4. Dr. Reddy's 23-Mar-10 MF-246/10 dialysis and for the treatment 2 Darbepoetin alfa Each 0.4 mL of single dose prefilled syringe Laboratories Ltd. 20-Jul-10 BULK-668/10 of anaemia in patients with contains 40mcg and 200mcg 5. Each 0.42 mL of non-myeloid malignancies, single dose prefilled syringe contains 25mcg 6. Each where anaemia is due to the 0.5 mL of single dose prefilled syringe contains effect of concomitantly 100mcg 7. Each 0.6 mL of single dose prefilled administered chemotherapy syringe contains 300mcg 8. Each 1 mL of single dose prefilled syringe contains 500mcg Gennova For prevention of neutropenia Pegfilgrastim Prefilled syringe for injection contains Pegfilgrastim 3 Biopharmaceuticals 29-Jan-10 MF-104/10 in the patient receiving cancer (peg-r-hu-GCSF) 6 mg/0.6 mL Ltd. -

AHS Provincial High-Alert Medication List
Provincial High-alert Medication List Classes/Categories of Medications Specific Medications adrenergic agonists: IV (e.g., epiNEPHrine, ePHEDrine, isoproterenol, magnesium sulfate injection PHENYLephrine, norepinephrine, doBUTamine, doPAMine, salbutamol) methotrexate: oral for non-oncologic adrenergic antagonists: IV (e.g., propranolol, metoPROLOL, labetalol, use esmolol) nitroprusside sodium for injection anesthetic agents: general, inhaled and IV (e.g., propofol, ketamine, sevoflurane, isoflurane, desflurane, etomidate) opium tincture antiarrhythmics: IV (e.g., lidocaine, amiodarone, procainamide, oxytocin: IV adenosine, bretylium, ibutilide) potassium chloride for injection: antithrombotic agents: concentrate anticoagulants (e.g., warfarin, acenocoumarol, tinzaparin, potassium phosphates injection enoxaparin, dalteparin, danaparoid, unfractionated heparin, sodium citrate) promethazine: IV factor Xa inhibitors (e.g., fondaparinux, rivaroxaban) vasopressin: IV or intraosseous direct thrombin inhibitors (e.g., apixaban, argatroban, bivalirudin, dabigatran) thrombolytics (e.g., alteplase, tenecteplase) glycoprotein IIb/IIIa inhibitors (e.g.,eptifibitide, tirofiban, abciximab) cardioplegic solutions chemotherapeutic agents: parenteral and oral dextrose: 20% or greater (hypertonic) dialysis solutions: peritoneal and hemodialysis The following medications are also epidural or intrathecal medications associated with higher risk and must inotropic medications: IV (e.g., digoxin, milrinone) comply with the segregated storage and labelling