Argatroban for Anticoagulation in Continuous Renal Replacement Therapy*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Thrombin and Thromboxane A2 in Reocclusion Following Coronary
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 7585-7589, October 1989 Medical Sciences Role of thrombin and thromboxane A2 in reocclusion following coronary thrombolysis with tissue-type plasminogen activator (thrombolytic therapy/coronary thrombosis/platelet activation/reperfusion) DESMOND J. FITZGERALD*I* AND GARRET A. FITZGERALD* Divisions of *Clinical Pharmacology and tCardiology, Vanderbilt University, Nashville, TN 37232 Communicated by Philip Needleman, June 28, 1989 (receivedfor review April 12, 1989) ABSTRACT Reocclusion of the coronary artery occurs against the prothrombinase formed on the platelet surface after thrombolytic therapy of acute myocardlal infarction (13) and the neutralization ofheparin by platelet factor 4 (14) despite routine use of the anticoagulant heparin. However, and thrombospondin (15), proteins released by activated heparin is inhibited by platelet activation, which is greatly platelets. enhanced in this setting. Consequently, it is unclear whether To address the role of thrombin during coronary throm- thrombin induces acute reocclusion. To address this possibility, bolysis, we have examined the effect of a specific thrombin we examined the effect of argatroban {MCI9038, (2R,4R)- inhibitor, argatroban {MCI9038, (2R,4R)-4-methyl-1-[N-(3- 4-methyl-l-[Na-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfo- methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl]-2- nyl)-L-arginyl]-2-piperidinecarboxylic acid}, a specific throm- piperidinecarboxylic acid} on the response to tissue plasmin- bin inhibitor, on the response to tissue-type plasminogen ogen activator (t-PA) in a closed-chest canine model of activator in a dosed-chest canine model of coronary thrombo- coronary thrombosis. MCI9038, an argimine derivative which sis. MCI9038 prolonged the thrombin time and shortened the binds to a hydrophobic pocket close to the active site of time to reperfusion (28 + 2 min vs. -

Stroke Prevention in Chronic Kidney Disease Disclosures
5/18/2020 Controversies: Stroke Prevention in Chronic Kidney Disease Wei Ling Lau, MD FASN FAHA FACP Assistant Professor, Nephrology University of California, Irvine Visiting Fellow at OptumLabsCOPY Disclosures • Prior or current research funding from NIH, AHA, Sanofi, ZS Pharma, and Hub Therapeutics. • Associate Medical Director for home peritoneal dialysis at Fresenius University Dialysis Center of Orange. • Has beenNOT on Fresenius medical advisory board for Velphoro. • No conflicts of interest relevant to the current talk. Controversies: Stroke prevention in CKD Wei Ling Lau, MD DO 1 5/18/2020 Stroke Prevention in CKD • Blood pressure targets • Antiplatelet agents • Statins • Anticoagulation Controversies: Stroke prevention in CKD Wei Ling Lau, MD COPY BP TARGETS Data is limited, as patients with CKD were historically excluded from clinical trials NOT Whelton 2017 ACC/AHA hypertension guidelines [Hypertension 2018] Controversies: Stroke prevention in CKD Wei Ling Lau, MD DO 2 5/18/2020 Systolic Blood Pressure Intervention Trial SPRINT: BP lowering to <120 vs <140 mmHg significantly lowered rate of CVD composite primary outcome; no clear effect on stroke Controversies: Stroke prevention in CKD The SPRINT Research Group. N Engl J Med 2015 p2103 Wei Ling Lau, MD COPY SPRINT subgroup analysis: CKD • Patients with CKD stage 3‐4 (eGFR of 20 to <60) comprised 28% of the SPRINT study population • Intensive BP management seemed to provide the same benefits for reduction in the CVD composite primary outcomeNOT – but did not impact stroke Controversies: Stroke prevention in CKD Cheung 2017 J Am Soc Nephrol p2812 Wei Ling Lau, MD DO 3 5/18/2020 The hazard of incident stroke associated with systolic BP (SBP) and chronic kidney disease (CKD)BP using and an unadjusted stroke model risk: that contained J‐shaped dummy variables association for CKD and BP groups (A) and a fully adjusted model that contained dummy variables for CKD and BP grou.. -

GTH 2021 State of the Art—Cardiac Surgery: the Perioperative Management of Heparin-Induced Thrombocytopenia in Cardiac Surgery
Review Article 59 GTH 2021 State of the Art—Cardiac Surgery: The Perioperative Management of Heparin-Induced Thrombocytopenia in Cardiac Surgery Laura Ranta1 Emmanuelle Scala1 1 Department of Anesthesiology, Cardiothoracic and Vascular Address for correspondence Emmanuelle Scala, MD, Centre Anesthesia, Lausanne University Hospital (CHUV), Lausanne, Hospitalier Universitaire Vaudois, Rue du Bugnon 46, BH 05/300, 1011 Switzerland Lausanne, Suisse, Switzerland (e-mail: [email protected]). Hämostaseologie 2021;41:59–62. Abstract Heparin-induced thrombocytopenia (HIT) is a severe, immune-mediated, adverse drug Keywords reaction that paradoxically induces a prothrombotic state. Particularly in the setting of ► Heparin-induced cardiac surgery, where full anticoagulation is required during cardiopulmonary bypass, thrombocytopenia the management of HIT can be highly challenging, and requires a multidisciplinary ► cardiac surgery approach. In this short review, the different perioperative strategies to run cardiopul- ► state of the art monary bypass will be summarized. Introduction genicity of the antibodies and is diagnostic for HIT. The administration of heparin to a patient with circulating Heparin-induced thrombocytopenia (HIT) is a severe, im- pathogenic HITabs puts the patient at immediate risk of mune-mediated, adverse drug reaction that paradoxically severe thrombotic complications. induces a prothrombotic state.1,2 Particularly in the setting The time course of HIT can be divided into four distinct of cardiac surgery, where full anticoagulation is required phases.6 Acute HIT is characterized by thrombocytopenia during cardiopulmonary bypass (CPB), the management of and/or thrombosis, the presence of HITabs, and confirma- HIT can be highly challenging, and requires a multidisciplin- tion of their platelet activating capacity by a functional ary approach. -

Statistical Analysis Plan – Part 1
NCT03251482 Janssen Research & Development Statistical Analysis Plan – Part 1 A Randomized, Double-blind, Double-dummy, Multicenter, Adaptive Design, Dose Escalation (Part 1) and Dose-Response (Part 2) Study to Evaluate the Safety and Efficacy of Intravenous JNJ-64179375 Versus Oral Apixaban in Subjects Undergoing Elective Total Knee Replacement Surgery Protocol 64179375THR2001; Phase 2 JNJ-64179375 Status: Approved Date: 18 October 2017 Prepared by: Janssen Research & Development, LLC Document No.: EDMS-ERI-148215770 Compliance: The study described in this report was performed according to the principles of Good Clinical Practice (GCP). Confidentiality Statement The information in this document contains trade secrets and commercial information that are privileged or confidential and may not be disclosed unless such disclosure is required by applicable law or regulations. In any event, persons to whom the information is disclosed must be informed that the information is privileged or confidential and may not be further disclosed by them. These restrictions on disclosure will apply equally to all future information supplied to you that is indicated as privileged or confidential. 1 Approved, Date: 18 October 2017 JNJ-64179375 NCT03251482 Statistical Analysis Plan - Part 1 64179375THR2001 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................... 2 LIST OF IN-TEXT TABLES AND FIGURES ............................................................................................... -

Interactions with COUMADIN
Interactions with COUMADIN INDICATIONS • have kidney problems • take other medicines that increase your risk of COUMADIN® (warfarin sodium) is a prescription medicine used bleeding, including: to treat blood clots and to lower the chance of blood clots – a medicine that contains heparin forming in your body. Blood clots can cause a stroke, heart – other medicines to prevent or treat blood clots attack, or other serious conditions if they form in the legs or – non-steroidal anti-inflammatory drugs (NSAIDs) lungs. COUMADIN may have been prescribed to help you: • take warfarin sodium for a long time. Warfarin sodium is • Reduce your risk of forming blood clots if you have had a the active ingredient in COUMADIN. heart-valve replacement or if you have an irregular, rapid Call your doctor or seek immediate medical care heartbeat, called atrial fibrillation if you have any of the following signs or symptoms • Lower the risk of death if you’ve had a heart attack, as of bleeding: well as lowering your risk of having another heart attack, • pain, swelling, or discomfort stroke, and having blood clots move to another part of • headaches, dizziness, or weakness your body • unusual bruising (bruises that develop without known It is not known if COUMADIN is safe and effective in children. cause or grow in size) • nosebleeds or bleeding gums IMPORTANT SAFETY INFORMATION • bleeding from cuts takes a long time to stop • menstrual bleeding or vaginal bleeding that is heavier COUMADIN® (warfarin sodium) can cause bleeding than normal which can be serious -

Anticoagulation Protocol
ANTICOAGULATION PROTOCOL Federal Bureau of Prisons Clinical Guidance MARCH 2018 This Anticoagulation Protocol is made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant this guidance for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient specific. Consult the BOP Clinical Guidance Web page to determine the date of the most recent update to this document: http://www.bop.gov/resources/health_care_mngmt.jsp Federal Bureau of Prisons Anticoagulation Protocol Clinical Guidance March 2018 WHAT’S NEW IN THIS DOCUMENT? The following changes have been made to the BOP Anticoagulation Protocol since it was last issued in April 2013: • A new table has been added to Section 4, Heparin Products. See Table 2. Dosing of LMWHs for Treatment and Prevention. • In Section 5, Warfarin, the discussion has been expanded to include lifestyle factors and health conditions affecting warfarin therapy. See Interactions with Food, Drugs, Lifestyle, and Health Conditions. • A new Section 6 on Novel Oral Anticoagulants (NOACs) has been added. • Information on Treatment of Deep Venous Thrombosis (DVT) and Pulmonary Embolism (PE) has been updated in Section 7. • The Inmate Fact Sheet on Warfarin is now available in Spanish. See Appendix 9B. • The CHA2DS2-VASc score has replaced the CHADS2 score for predicting thromboembolic stroke risk in non-valvular atrial fibrillation. See Appendix 10, Table B. i Federal Bureau of Prisons Anticoagulation Protocol Clinical Guidance March 2018 TABLE OF CONTENTS 1. -

AHS Provincial High-Alert Medication List
Provincial High-alert Medication List Classes/Categories of Medications Specific Medications adrenergic agonists: IV (e.g., epiNEPHrine, ePHEDrine, isoproterenol, magnesium sulfate injection PHENYLephrine, norepinephrine, doBUTamine, doPAMine, salbutamol) methotrexate: oral for non-oncologic adrenergic antagonists: IV (e.g., propranolol, metoPROLOL, labetalol, use esmolol) nitroprusside sodium for injection anesthetic agents: general, inhaled and IV (e.g., propofol, ketamine, sevoflurane, isoflurane, desflurane, etomidate) opium tincture antiarrhythmics: IV (e.g., lidocaine, amiodarone, procainamide, oxytocin: IV adenosine, bretylium, ibutilide) potassium chloride for injection: antithrombotic agents: concentrate anticoagulants (e.g., warfarin, acenocoumarol, tinzaparin, potassium phosphates injection enoxaparin, dalteparin, danaparoid, unfractionated heparin, sodium citrate) promethazine: IV factor Xa inhibitors (e.g., fondaparinux, rivaroxaban) vasopressin: IV or intraosseous direct thrombin inhibitors (e.g., apixaban, argatroban, bivalirudin, dabigatran) thrombolytics (e.g., alteplase, tenecteplase) glycoprotein IIb/IIIa inhibitors (e.g.,eptifibitide, tirofiban, abciximab) cardioplegic solutions chemotherapeutic agents: parenteral and oral dextrose: 20% or greater (hypertonic) dialysis solutions: peritoneal and hemodialysis The following medications are also epidural or intrathecal medications associated with higher risk and must inotropic medications: IV (e.g., digoxin, milrinone) comply with the segregated storage and labelling -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -
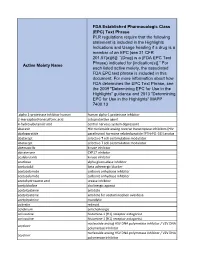
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Study Protocol
Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Synopsis Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Assess the Safety and Efficacy of ART-123 in Subjects with Severe Sepsis and Coagulopathy Name of Sponsor/Company: Asahi Kasei Pharma America Corporation Name of Investigational Product: ART-123 Name of Active Ingredient: thrombomodulin alpha Objectives Primary: x To evaluate whether ART-123, when administered to subjects with bacterial infection complicated by at least one organ dysfunction and coagulopathy, can reduce mortality. x To evaluate the safety of ART-123 in this population. Secondary: x Assessment of the efficacy of ART-123 in resolution of organ dysfunction in this population. x Assessment of anti-drug antibody development in subjects with coagulopathy due to bacterial infection treated with ART-123. Study Center(s): Phase of Development: Global study, up to 350 study centers Phase 3 Study Period: Estimated time of first subject enrollment: 3Q 2012 Estimated time of last subject enrollment: 3Q 2018 Number of Subjects (planned): Approximately 800 randomized subjects. Page 2 of 116 Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Diagnosis and Main Criteria for Inclusion of Study Subjects: This study targets critically ill subjects with severe sepsis requiring the level of care that is normally associated with treatment in an intensive care unit (ICU) setting. The inclusion criteria for organ dysfunction and coagulopathy must be met within a 24 hour period. 1. Subjects must be receiving treatment in an ICU or in an acute care setting (e.g., Emergency Room, Recovery Room). -

Standardization of Intravenous Medication Beyond Use Dating at a Large Health-System
Title Page Standardization of intravenous medication beyond use dating at a large health-system by Margaret J. Kronz PharmD, Lake Erie College of Osteopathic Medicine, 2019 Submitted to the Graduate Faculty of the Graduate School of Public Health in partial fulfillment of the requirements for the degree of Master of Public Health University of Pittsburgh 2021 Committee Membership Page UNIVERSITY OF PITTSBURGH GRADUATE SCHOOL OF PUBLIC HEALTH This essay was submitted by Margaret J. Kronz It was defended on April 30, 2021 and approved by David Finegold, MD, MPH, Director, Multidisciplinary Master of Public Health Tina Batra Hershey, JD, MPH, Assistant Professor, Health Policy and Management Arpit Mehta, PharmD, MPH, MHA, Director of Pharmacy, Allegheny General Hospital Thesis Advisor: David Finegold MD, MPH, Director, Multidisciplinary Master of Public Health ii Copyright © by Margaret J. Kronz 2021 iii Abstract Standardization of intravenous medication beyond use dating at a large health-system Margaret J. Kronz, PharmD, MPH University of Pittsburgh, 2021 Abstract Within a large, nine-hospital health-system, there are a number of hospitals that have historically reviewed the current literature individually to establish hospital-specific non- hazardous compounded sterile product (CSP) beyond-use date (BUD) lists. Creating and standardizing the list on a system level is a potential model that could reduce work at the individual site level, increase network patient safety, and facilitate the implementation of network technology. Researchers reviewed the most up-to-date literature and current non-hazardous CSP BUD lists at each site for inconsistencies to highlight the value and increased safety associated with standardizing this resource across the network. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational