Reportable Neoplasms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

TUMOR and STAGING DATA Primary Site Code
SECTION IV - TUMOR and STAGING DATA Primary Site Code NAACCR Version 11.1 field "Primary Site", Item 400, columns 291-294 It is unclear how the 2007 MP/H rules may alter rules for assigning the best Primary Site Formatted: Left Code to each primary. Continue to use the following rules until new rules are issued. Enter the code for the site of origin from the Topography section of ICD-O-3. [Note that ICD-O-2 code C14.1, laryngopharynx, should not be used for diagnoses made on or after January 1, 1995. "Laryngopharynx" became an equivalent term under C13.9 (hypopharynx, NOS) as of this diagnosis date. Code C14.1 is not an ICD-O-3 code.] Enter the site code that matches the narrative primary site indicated in the medical record, or the site code most appropriate for the case. Site codes are found in ICD-O-3's Numerical Lists - Topography section (pages 45-65) and in its Alphabetic Index (pages 105-218). In ICD-O-3 primary site codes consist of the letter "C" followed by two digits, a decimal point, and a third digit. "C" should be entered but the decimal point should not be entered. Example: The primary site is "cardia of stomach". Look this up in the Alphabetic Index of ICD-O-3 under "stomach" or "cardia", and the corresponding code "C16.0" is found. Enter C160. Most sites include a third digit of "8" to be used for single tumors that overlap the boundaries of two or more anatomically contiguous subsites and whose exact point of origin cannot be determined, unless the combination of sites is specifically indexed elsewhere. -

2018 SEER Solid Tumor Manual
4/6/2018 Eight Groups are Revised for 2018 Head & Neck Colon (includes rectosigmoid and rectum for cases diagnosed 1/1/2018 forward) 2018 SEER Lung (2018 Draft not yet available) Breast Solid Tumor Manual Kidney Urinary Sites (2018 Draft not yet available) 2018 KCR SPRING TRAINING Non‐malignant CNS (2018 Draft not yet available) Malignant CNS and Peripheral Nerves (2018 Draft not yet available) 2019 Changes for other two Groups Solid Tumor Rules What we will cover: ◦ Overview of General rules Cutaneous melanoma (minor revisions and draft available now) • Cutaneous melanoma site rules will be revised for 2019 implementation to incorporate ◦ New Head and Neck rules information from the new WHO 4th Ed Tumors of Skin scheduled to be released in 2018. ◦ New Colorectal rules ◦ New Breast rules Other sites (minor revisions and draft available now) ◦ New Kidney rules • Primary sites excluded are: •Rectosigmoid and rectum which are included in 2018 Colon rules. •Peripheral nerves which are included in 2018 Malignant Brain rules. •Other sites rules will be revised for 2019 implementation. The Solid Tumor Task Force has identified Remember: These are currently in draft form and may change slightly in the final version! the need to expand the rules to include GYN, soft tissue, thyroid and other site‐specific solid tumors. NEW! Code subtypes/variants when definitively described (with no modifiers) General Instructions Example: Well ‐differentiated neuroendocrine tumor 8240. Do not code a histology (*including subtypes or variants) when described as below: The 2018 solid tumor rules replace all previous MP/H rules, but they are effective for diagnoses on or after 1/1/2018. -
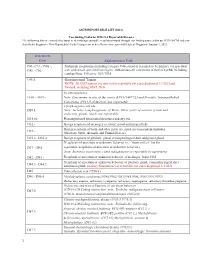
ASCR Reportable List ICD-10 Version
ASCR REPORTABLE LIST (2021) Casefinding Codes for ICD-O-3 Reportable Diseases The following lists are intended to assist in identifying reportable neoplasms found through casefinding sources that use ICD-10-CM codes to classify the diagnoses. New Reportable/ Code Changes are to be effective for cases with Date of Diagnosis January 1, 2021. ICD-10-CM Code Explanation of Code C00.- C43.-, C4A.-, Malignant neoplasms (excluding category C44), stated or resumed to be primary (of specified C45 - C96.- site) and certain specified histologies. Sebaceous cell carcinoma of skin of eyelid, including canthus Note: Effective 10/1/2018 C49.A- Gastrointestinal Tumors NOTE: All GIST tumors are now newly reportable for cases diagnosed 1/1/2021and forward, including GIST, NOS In-situ neoplasms D 00.- -D 09.- Note: Carcinoma in situ of the cervix (CIN 111-8077/2) and Prostatic lntraepithelial Carcinoma {PIN 111-8148/2) are not reportable Lymphangioma,any site D18.1 Note: Includes Lymphangiomas of Brain, Other parts of nervous system and endocrine glands, which are reportable D18.02 Hemangioma of intracranial structures and any site D32.- Benign neoplasm of meninges (cerebral, spinal and unspecified) Benign neoplasm of brain and other parts of central nervous system (includes D33.- Olfactory, Optic, Acoustic and Cranial Nerves) D35.2- D 35.4 Benign neoplasm of pituitary gland, craniopharyngeal duct and pineal gland Neoplasms of uncertain or unknown behavior (see "must collect" list for D37. - D41._ reportable neoplasms of uncertain or unknown behavior) Note: -

Duodenal Carcinomas
Modern Pathology (2017) 30, 255–266 © 2017 USCAP, Inc All rights reserved 0893-3952/17 $32.00 255 Non-ampullary–duodenal carcinomas: clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas Yue Xue1,9, Alessandro Vanoli2,9, Serdar Balci1, Michelle M Reid1, Burcu Saka1, Pelin Bagci1, Bahar Memis1, Hyejeong Choi3, Nobuyike Ohike4, Takuma Tajiri5, Takashi Muraki1, Brian Quigley1, Bassel F El-Rayes6, Walid Shaib6, David Kooby7, Juan Sarmiento7, Shishir K Maithel7, Jessica H Knight8, Michael Goodman8, Alyssa M Krasinskas1 and Volkan Adsay1 1Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, USA; 2Department of Molecular Medicine, San Matteo Hospital, University of Pavia, Pavia, Italy; 3Department of Pathology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea; 4Department of Pathology, Showa University Fujigaoka Hospital, Yokohama, Japan; 5Department of Pathology, Tokai University Hachioji Hospital, Tokyo, Japan; 6Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; 7Department of Surgery, Emory University School of Medicine, Atlanta, GA, USA and 8Department of Epidemiology, Emory University Rollins School of Public Health, Atlanta, GA, USA Literature on non-ampullary–duodenal carcinomas is limited. We analyzed 47 resected non-ampullary–duodenal carcinomas. Histologically, 78% were tubular-type adenocarcinomas mostly gastro-pancreatobiliary type and only 19% pure intestinal. Immunohistochemistry (n = 38) revealed commonness of ‘gastro-pancreatobiliary markers’ (CK7 55, MUC1 50, MUC5AC 50, and MUC6 34%), whereas ‘intestinal markers’ were relatively less common (MUC2 36, CK20 42, and CDX2 44%). Squamous and mucinous differentiation were rare (in five each); previously, unrecognized adenocarcinoma patterns were noted (three microcystic/vacuolated, two cribriform, one of comedo-like, oncocytic papillary, and goblet-cell-carcinoid-like). -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

Loss of Heterozygosity in Human Ductal Breast Tumors Indicates a Recessive Mutation on Chromosome 13
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 2372-2376, April 1987 Genetics Loss of heterozygosity in human ductal breast tumors indicates a recessive mutation on chromosome 13 (carcinogenesis/mapping/somatic mutations/DNA polymorphisms) CATHARINA LUNDBERG*, LAMBERT SKOOGt, WEBSTER K. CAVENEEt, AND MAGNUS NORDENSKJOLD*§ Departments of *Clinical Genetics and tTumor Pathology, Karolinska Hospital, S-10401 Stockholm, Sweden; and tLudwig Institute for Cancer Research, Royal Victoria Hospital and McGill University, Montreal, Quebec H3A lAl, Canada Communicated by RolfLuft, December 3, 1986 ABSTRACT The genotypes at chromosomal loci defined tumor, hepatoblastoma, and rhabdomyosarcoma, for which by recombinant DNA probes revealing restriction fragment specific rearrangements involving the short arm ofchromosome length polymorphisms were determined in constitutional and 11 were demonstrated (14). Cases of these tumors sometimes tumor tissue from 10 cases of ductal breast cancer: eight show familial clustering as one manifestation of the autosomal premenopausal females and two males. Somatic loss of consti- dominant Beckwith-Wiedemann syndrome (5), which has also tutional heterozygosity was observed at loci on chromosome 13 been regionally mapped to lip (15), or have been observed in primary tumor tissue from three females and one male. In simultaneously as heterotropic tumors. two cases, specific loss ofheterozygosity at three distinct genetic These studies have presented experimental evidence in loci along the length of the chromosome was observed. In support of the two-step hypothesis for tumorigenesis by another case, concurrent loss of alleles at loci on chromosomes Knudson (6). They indicate that sporadic and inherited forms 2, 13, 14, and 20 was detected, whereas a fourth case showed of embryonal tumors affect the same loci and that the tumors loss of heterozygosity for chromosomes 5 and 13. -

Breast Histology Coding Rules Text Format
Breast Histology Coding Rules – Text C500-C509 (Excludes lymphoma and leukemia M9590-9989 and Kaposi sarcoma M9140) SINGLE TUMOR: IN SITU CARCINOMA ONLY (Single Tumor; all parts are in situ) Rule H1 Code the histology documented by the physician when the pathology/cytology report is not available. Note 1: Priority for using documents to code the histology • Documentation in the medical record that refers to pathologic or cytologic findings • Physician’s reference to type of cancer (histology) in the medical record Note 2: Code the specific histology when documented. Rule H2 Code the histology when only one histologic type is identified Rule H3 Code the more specific histologic term when the diagnosis is: • Carcinoma in situ, NOS (8010) and a specific carcinoma in situ or • Adenocarcinoma in situ, NOS (8140) and a specific adenocarcinoma in situ or • Intraductal carcinoma, NOS (8500) and a specific intraductal carcinoma (Table 1) Note: The specific histology may be identified as type, subtype, predominantly, with features of, major, with ___ differentiation, architecture or pattern. The terms architecture and pattern are subtypes only for in situ cancer. Rule H4 Code 8501/2 (comedocarcinoma, non-infiltrating) when there is non-infiltrating comedocarcinoma and any other intraductal carcinoma (Table 1). Example: Pathology report reads intraductal carcinoma with comedo and solid features. Code 8501/2 (comedocarcinoma). Rule H5 Code 8522/2 (intraductal carcinoma and lobular carcinoma in situ) (Table 3) when there is a combination of in situ lobular (8520) and intraductal carcinoma (Table 1). Rule H6 Code 8523/2 (intraductal carcinoma mixed with other types of in situ carcinoma) (Table 3) when there is a combination of intraductal carcinoma and two or more specific intraductal types OR there are two or more specific intraductal carcinomas. -

Effective for Cases Diagnosed January 1, 2016 and Later
POLICY AND PROCEDURE MANUAL FOR REPORTING FACILITIES May 2016 Effective For Cases Diagnosed January 1, 2016 and Later Indiana State Cancer Registry Indiana State Department of Health 2 North Meridian Street, Section 6-B Indianapolis, IN 46204-3010 TABLE OF CONTENTS INDIANA STATE DEPARTMENT OF HEALTH STAFF ............................................................................. viii INDIANA STATE DEPARTMENT OF HEALTH CANCER REGISTRY STAFF .......................................... ix ACKNOWLEDGMENTS ................................................................................................................................ x INTRODUCTION ........................................................................................................................................... 1 A. Background ..................................................................................................................................... 1 B. Purpose .......................................................................................................................................... 1 C. Definitions ....................................................................................................................................... 1 D. Reference Materials........................................................................................................................ 1 E. Consultation .................................................................................................................................... 2 F. Output ............................................................................................................................................ -

Understanding a Breast Cancer Diagnosis Breast Cancer Grade and Other Tests
cancer.org | 1.800.227.2345 Understanding a Breast Cancer Diagnosis Breast Cancer Grade and Other Tests Doctors use information from your breast biopsy to learn a lot of important things about the exact kind of breast cancer you have. ● Breast Cancer Grades ● Breast Cancer Ploidy and Cell Proliferation ● Breast Cancer Hormone Receptor Status ● Breast Cancer HER2 Status ● Breast Cancer Gene Expression Tests ● Understanding Your Pathology Report Stages and Outlook (Prognosis) If you have been diagnosed with breast cancer, tests will be done to find out the extent (stage) of the cancer. The stage of a cancer helps determine how serious the cancer is and how best to treat it. ● Imaging Tests to Find Out if Breast Cancer Has Spread ● Breast Cancer Stages ● Breast Cancer Survival Rates Questions to Ask About Your Breast Cancer You can take an active role in your breast cancer care by learning about your cancer and its treatment and by asking questions. Get a list of key questions here. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● Questions to Ask Your Doctor About Breast Cancer Connect with a breast cancer survivor Reach To Recovery The American Cancer Society Reach To Recovery® program connects people facing breast cancer – from diagnosis through survivorship – with trained volunteers who are breast cancer survivors. Our volunteers provide one-on-one support through our website and mobile app to help those facing breast cancer cope with diagnosis, treatment, side effects, and more. Breast Cancer Grades Knowing a breast cancer’s grade is important to understand how fast it’s likely to grow and spread. -

Statistical Reflections Regarding the Significance of the Microscopic Examination in the Diagnosis of Mammary and Abdominal Neoplasias in Cats
SHORT COMMUNICATIONS Statistical Reflections Regarding the Significance of the Microscopic Examination in the Diagnosis of Mammary and Abdominal Neoplasias in Cats 1 * 1 FacultyElena GAVRILAȘ of Veterinary, Vasile Medicine VULPE of Iasi, Ion Ionescu de la Brad University of Agricultural Sciences and Veterinary Medicine, Mihail Sadoveanu Street, no.8, 700489 *corresponding author: [email protected] Bulletin UASVM Veterinary Medicine2018.0022 76(1)/2019 Print ISSN 1843-5270; Electronic ISSN 1843-5378 doi:10.15835/buasvmcn-vm: Abstract 76 cats were clinical examined for mammary and abdominal neoplasias. In 41 cats were performed microscopic examination. Regarding the location of the primary tumors 70 cats had mammary tumors. Isolated, six cases of primary non-mammary tumors were diagnosed following necropsy and histopathology. Following cytopathological and histopathological examinations, 14 tumor types were identified, of which 3 benign and 11 malignant. Benign tumoral types consisted of lipoma, vesical leiomyoma and mammary adenoma. Diagnosed malignant cases consisted of simple mammary adenocarcinoma, solid adenocarcinoma, compact adenocarcinoma, hepatic cholangiocarcinoma, compact carcinoma, mixed pulmonar bronchioloalveolar carcinoma, hemangiosarcoma, mammaryKeywords: comedocarcinoma, solid carcinoma and mixed adenocarcinoma. cat, examination, mammary, microscopic, neoplasias Introduction being studied,et al. and it is one of the major morbidity The importance of the neoplastic disease, and mortalityet al causes in cats and dogs (Barbara, -

Comparative Analysis of the Histopathological
ORIGINAL ARTICLE J Bras Patol Med Lab. 2019; 55(1): 69-86. Comparative analysis of the histopathological and epidemiological profile of ductal and lobular breast carcinomas diagnosed at the Hospital de Clínicas da Universidade Federal do Paraná during 10.5935/1676-2444.20190009 the period 2008-2013 Análise comparativa do perfil histopatológico e epidemiológico dos carcinomas ductal e lobular da mama diagnosticados no Hospital de Clínicas da Universidade Federal do Paraná entre 2008 e 2013 Heloisa Z. Rocha; Graciele C. M. Manica; Lucia de Noronha; Edneia A. S. Ramos; Giseli Klassen Universidade Federal do Paraná (UFPR), Curitiba, Paraná, Brazil. ABSTRACT Introduction: Breast cancer is the second leading cause of cancer death among women worldwide, and epidemiological studies may help understanding its mechanisms. Objective: To carry out a survey of the number of breast cancer cases diagnosed in a period of six years. Methods: The profile of breast cancers diagnosed in a tertiary hospital in Curitiba was compared with the literature, using a retrospective analysis of ductal/special types and lobular breast carcinoma reports issued between 2008 and 2013. Results: Three hundred twenty-seven (91.6%) cases of ductal/special types carcinoma and 30 (8.4%) cases of lobular carcinoma were diagnosed, totaling 357 samples. From these cases, 27 (7.5%) were carcinoma in situ (20 ductal and seven lobular) and 330 (92.4%) were invasive carcinoma (307 invasive ductal/special types and 23 lobular). The prevalence of breast cancer among women was 99.1% and the majority of patients were older than 50 years of age (67.2%). Regarding the União Internacional de Controle do Câncer/American Joint Committee on Cancer (UICC/AJCC) staging, 49.2% of the ductal/special types tumors were diagnosed in Stages I or II, while 56.6% of lobular carcinomas were diagnosed in Stages II or III/IV.