Hybrid Oaks (Quercus Spp.) of Georgia K.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Lake Harney Wilderness Area Land Management Plan
Lake Harney Wilderness Area Land Management Plan 2010 LAKE HARNEY WILDERNESS AREA LAND MANAGEMENT PLAN TABLE OF CONTENTS INTRODUCTION ..................................................................................................................................................... 1 WILDERNESS AREA OVERVIEW .............................................................................................................................. 1 REGIONAL SIGNIFICANCE .................................................................................................................................................. 1 ACQUISITION HISTORY ..................................................................................................................................................... 3 NATURAL RESOURCES OVERVIEW ......................................................................................................................... 4 NATURAL COMMUNITIES.................................................................................................................................................. 4 FIRE ............................................................................................................................................................................. 5 WILDLIFE ...................................................................................................................................................................... 5 EXOTICS ....................................................................................................................................................................... -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Oaks (Quercus Spp.): a Brief History
Publication WSFNR-20-25A April 2020 Oaks (Quercus spp.): A Brief History Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Quercus (oak) is the largest tree genus in temperate and sub-tropical areas of the Northern Hemisphere with an extensive distribution. (Denk et.al. 2010) Oaks are the most dominant trees of North America both in species number and biomass. (Hipp et.al. 2018) The three North America oak groups (white, red / black, and golden-cup) represent roughly 60% (~255) of the ~435 species within the Quercus genus worldwide. (Hipp et.al. 2018; McVay et.al. 2017a) Oak group development over time helped determine current species, and can suggest relationships which foster hybridization. The red / black and white oaks developed during a warm phase in global climate at high latitudes in what today is the boreal forest zone. From this northern location, both oak groups spread together southward across the continent splitting into a large eastern United States pathway, and much smaller western and far western paths. Both species groups spread into the eastern United States, then southward, and continued into Mexico and Central America as far as Columbia. (Hipp et.al. 2018) Today, Mexico is considered the world center of oak diversity. (Hipp et.al. 2018) Figure 1 shows genus, sub-genus and sections of Quercus (oak). History of Oak Species Groups Oaks developed under much different climates and environments than today. By examining how oaks developed and diversified into small, closely related groups, the native set of Georgia oak species can be better appreciated and understood in how they are related, share gene sets, or hybridize. -

Native Nebraska Woody Plants
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS NATIVE NEBRASKA WOODY PLANTS Trees (Genus/Species – Common Name) 62. Atriplex canescens - four-wing saltbrush 1. Acer glabrum - Rocky Mountain maple 63. Atriplex nuttallii - moundscale 2. Acer negundo - boxelder maple 64. Ceanothus americanus - New Jersey tea 3. Acer saccharinum - silver maple 65. Ceanothus herbaceous - inland ceanothus 4. Aesculus glabra - Ohio buckeye 66. Cephalanthus occidentalis - buttonbush 5. Asimina triloba - pawpaw 67. Cercocarpus montanus - mountain mahogany 6. Betula occidentalis - water birch 68. Chrysothamnus nauseosus - rabbitbrush 7. Betula papyrifera - paper birch 69. Chrysothamnus parryi - parry rabbitbrush 8. Carya cordiformis - bitternut hickory 70. Cornus amomum - silky (pale) dogwood 9. Carya ovata - shagbark hickory 71. Cornus drummondii - roughleaf dogwood 10. Celtis occidentalis - hackberry 72. Cornus racemosa - gray dogwood 11. Cercis canadensis - eastern redbud 73. Cornus sericea - red-stem (redosier) dogwood 12. Crataegus mollis - downy hawthorn 74. Corylus americana - American hazelnut 13. Crataegus succulenta - succulent hawthorn 75. Euonymus atropurpureus - eastern wahoo 14. Fraxinus americana - white ash 76. Juniperus communis - common juniper 15. Fraxinus pennsylvanica - green ash 77. Juniperus horizontalis - creeping juniper 16. Gleditsia triacanthos - honeylocust 78. Mahonia repens - creeping mahonia 17. Gymnocladus dioicus - Kentucky coffeetree 79. Physocarpus opulifolius - ninebark 18. Juglans nigra - black walnut 80. Prunus besseyi - western sandcherry 19. Juniperus scopulorum - Rocky Mountain juniper 81. Rhamnus lanceolata - lanceleaf buckthorn 20. Juniperus virginiana - eastern redcedar 82. Rhus aromatica - fragrant sumac 21. Malus ioensis - wild crabapple 83. Rhus copallina - flameleaf (shining) sumac 22. Morus rubra - red mulberry 84. Rhus glabra - smooth sumac 23. Ostrya virginiana - hophornbeam (ironwood) 85. Rhus trilobata - skunkbush sumac 24. Pinus flexilis - limber pine 86. Ribes americanum - wild black currant 25. -

Plant Blindness Identification
Plant Blindness Identification 1 broadleaf plantain Plantago major; introduced weed, but not harmful to local ecology; originally from Europe, now one of the most widely distributed plant species in the world; one of the first plants from Europe to establish populations in North America. Also known as white man’s footprint, for it seemed to follow European colonists everywhere they went. 1 white clover Trifolium repens; introduced weed, but not harmful to local ecology; much loved by honeybees 2 dandelion Taraxicum officinale; introduced weed, but not harmful to local ecology; distributed across the world, the leaves are edible 3 pokeweed Phytolacca americana; weedy native, very valuable plant for birds, who eat the berries; this plant is highly toxic to humans, both leaves and berries 4 daylily Hemerocallis (this one happens to be ‘Stella d’Oro’); introduced, not invasive; native to east Asia, daylilies have been cultivated for hundreds of years, with more than 80,000 different varieties 5 fern Some introduced ferns can be harmful invasives for local ecology; most ferns prefer shady areas with moist soil, although many will tolerate sun and some will grow in extremely dry conditions 6 mophead hydrangea Hydrangea macrophylla; introduced, not invasive; native to Japan, this is one of the most commonly used garden plants in the southeast; the flowers can be either pink or blue, depending on the acidity of the soil 7 nandina Nandina domestica; introduced, harmful invasive for local ecology; the berries are very toxic to birds; native to -

Ecological Summary Report
Ecological Summary Report 56.8-acre Ameratrail Property Section 14, Township 26 South, Range 31 East Osceola County, Florida Prepared for: Snow Construction, Inc. 1136 New York Avenue St Cloud, FL 34769 October 2018 Table of Contents 1.0 Property Location 1 2.0 Survey Methodology 1 3.0 Soils 1 4.0 Vegetative Communities 2 5.0 Listed Wildlife Species 3 5.1 Bald Eagle 3 5.2 Gopher Tortoise 3 5.3 Red Cockaded Woodpecker 3 5.4 Florida Scrub-Jay 3 5.5 Eastern Indigo Snake 3 6.0 Ecological Impact Analysis 4 7.0 Conclusion 4 List of Figures: Figure 1 – Location Map Figure 2 – Aerial Photograph Figure 3 – USDA Soils Map Figure 4 – FLUCFCS Map This report presents the results of an ecological impact analysis for the Ameratrail project. The purpose of this analysis is to document existing site conditions, and the extent of impacts to jurisdictional wetlands and surface waters, as well as state and federally-listed species and their habitats. This information is intended to support Environmental Resource Permitting through the South Florida Water Management District (SFWMD). 1.0 PROPERTY LOCATION The property consists of approximately 56.8 acres and is located southeast of the intersection of Old Melbourne Highway and US Highway 192 within Section 14, Township 26 South, Range 31 East, in Osceola County. The property consists of Osceola County tax parcel 14-26-31-0000-0010-0000. A location map and an aerial photograph have been provided as Figures 1 and 2, respectively. 2.0 SURVEY METHODOLOGY Prior to visiting the site, Austin Environmental Consultants, Inc. -

Quercus Coccinea.Indd
Quercus coccinea (Scarlet Oak) Beech Family (Fagaceae) Introduction: Scarlet oak is a member of the red oak group with lobed leaves and is valued for its ornamental attributes as well as its fi ne wood. It is considered an excellent alternative to the overplanted pin oak because it is beautiful throughout the year and tolerates alkaline soil. Culture: Scarlet oak makes an excellent lawn or park tree. It thrives in full sun and well-drained, acidic soil. This tree is less susceptible to chlorosis problems than pin oak. Potential problems on oaks in general include obscure scale, two-lined chestnut borer, bacterial leaf scorch, oak horn gall and gypsy moth. In addition, as little as 1 inch of fi ll soil can kill an oak. Botanical Characteristics: Additional information: Scarlet oak is appropriately named for its Native habit: Maine south to Florida, west to extraordinary leaf color in early spring and in autumn. Minnesota and Missouri. Its delicate branching pattern combined with nearly black bark and persistent leaves make this an exceptional tree Growth habit: Scarlet oak has a rounded, open even in winter. form at maturity. Although scarlet oak has a predominant tap root, it does have some spreading lateral roots, making the tree Tree size: This oak can attain a height of 70 to easier to transplant than some oaks. It is best to transplant 75 feet and a width of 40 to 50 feet. It can reach a the tree in the fall. The lateral roots may appear above the height of 100 feet in the wild. -

Species Profile: Quercus Oglethorpensis
Conservation Gap Analysis of Native U.S. Oaks Species profile: Quercus oglethorpensis Emily Beckman, Matt Lobdell, Abby Meyer, Murphy Westwood SPECIES OF CONSERVATION CONCERN CALIFORNIA SOUTHWESTERN U.S. SOUTHEASTERN U.S. Channel Island endemics: Texas limited-range endemics State endemics: Quercus pacifica, Quercus tomentella Quercus carmenensis, Quercus acerifolia, Quercus boyntonii Quercus graciliformis, Quercus hinckleyi, Southern region: Quercus robusta, Quercus tardifolia Concentrated in Florida: Quercus cedrosensis, Quercus dumosa, Quercus chapmanii, Quercus inopina, Quercus engelmannii Concentrated in Arizona: Quercus pumila Quercus ajoensis, Quercus palmeri, Northern region and / Quercus toumeyi Broad distribution: or broad distribution: Quercus arkansana, Quercus austrina, Quercus lobata, Quercus parvula, Broad distribution: Quercus georgiana, Quercus sadleriana Quercus havardii, Quercus laceyi Quercus oglethorpensis, Quercus similis Quercus oglethorpensis W.H.Duncan Synonyms: N/A Common Names: Oglethorpe oak Species profile co-author: Matt Lobdell, The Morton Arboretum Suggested citation: Beckman, E., Lobdell, M., Meyer, A., & Westwood, M. (2019). Quercus oglethorpensis W.H.Duncan. In Beckman, E., Meyer, A., Man, G., Pivorunas, D., Denvir, A., Gill, D., Shaw, K., & Westwood, M. Conservation Gap Analysis of Native U.S. Oaks (pp. 152-157). Lisle, IL: The Morton Arboretum. Retrieved from https://www.mortonarb.org/files/species-profile-quercus-oglethorpensis.pdf Figure 1. County-level distribution map for Quercus oglethorpensis. Source: Biota of North America Program (BONAP).4 Matt Lobdell DISTRIBUTION AND ECOLOGY Quercus oglethorpensis, or Oglethorpe oak, has a disjointed distribution across the southern U.S. Smaller clusters of localities exist in northeastern Louisiana, southeastern Mississippi, and southwestern Alabama, and a more extensive and well-known distribution extends from northeastern Georgia across the border into South Carolina. -
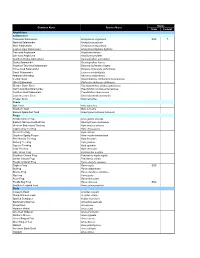
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

The Effect of Season of Fire on the Recovery of Florida Scrub
2B.7 THE EFFECT OF SEASON OF FIRE ON THE RECOVERY OF FLORIDA SCRUB Tammy E. Foster* and Paul A. Schmalzer Dynamac Corporation, Kennedy Space Center, Florida 1. ABSTRACT∗ scrub, rosemary scrub, and scrubby flatwoods (Myers 1990, Abrahamson and Hartnett 1990). Florida scrub is a xeromorphic shrubland that is Florida scrub is habitat for threatened and maintained by frequent fires. Historically, these fires endangered plants and animals (Christman and Judd occurred during the summer due to lightning ignition. 1990, Stout and Marion 1993, Stout 2001). Today, Florida scrub is often managed by the use of Management of remaining scrub is critical to the survival prescribed burning. Prescribed burning of scrub has of these species. Scrub is a fire-maintained system been implemented on Kennedy Space Center/Merritt (Myers 1990, Menges 1999), and recovery after fire of Island National Wildlife Refuge (KSC/MINWR) since oak scrub and scrubby flatwoods is primarily through 1981, with burns being carried out throughout the year. sprouting and, in some species, clonal spread of the The impacts of the season of burn on recovery are not dominant shrubs (Abrahamson 1984a, 1984b, known. Long-term monitoring of scrub regeneration has Schmalzer and Hinkle 1992a, Menges and Hawkes been conducted since the early-1980’s at KSC/MINWR 1998). Scrub naturally burned during the summer using permanent 15 m line-intercept transects. We months due to lightning ignition. However, landscape obtained data from eight transects that were subjected fragmentation and fire suppression have reduced fire to a winter burn in 1986 and a summer burn in 1997 and size and frequency (Myers 1990, Duncan and compared the recovery of the stand for the first five Schmalzer 2001). -

Key to Leaves of Eastern Native Oaks
FHTET-2003-01 January 2003 Front Cover: Clockwise from top left: white oak (Q. alba) acorns; willow oak (Q. phellos) leaves and acorns; Georgia oak (Q. georgiana) leaf; chinkapin oak (Q. muehlenbergii) acorns; scarlet oak (Q. coccinea) leaf; Texas live oak (Q. fusiformis) acorns; runner oak (Q. pumila) leaves and acorns; background bur oak (Q. macrocarpa) bark. (Design, D. Binion) Back Cover: Swamp chestnut oak (Q. michauxii) leaves and acorns. (Design, D. Binion) FOREST HEALTH TECHNOLOGY ENTERPRISE TEAM TECHNOLOGY TRANSFER Oak Identification Field Guide to Native Oak Species of Eastern North America John Stein and Denise Binion Forest Health Technology Enterprise Team USDA Forest Service 180 Canfield St., Morgantown, WV 26505 Robert Acciavatti Forest Health Protection Northeastern Area State and Private Forestry USDA Forest Service 180 Canfield St., Morgantown, WV 26505 United States Forest FHTET-2003-01 Department of Service January 2003 Agriculture NORTH AMERICA 100th Meridian ii iii ACKNOWLEDGMENTS The authors wish to thank all those who helped with this publication. We are grateful for permission to use the drawings illustrated by John K. Myers, Flagstaff, AZ, published in the Flora of North America, North of Mexico, vol. 3 (Jensen 1997). We thank Drs. Cynthia Huebner and Jim Colbert, U.S. Forest Service, Northeastern Research Station, Disturbance Ecology and Management of Oak-Dominated Forests, Morgantown, WV; Dr. Martin MacKenzie, U.S. Forest Service, Northeastern Area State and Private Forestry, Forest Health Protection, Morgantown, WV; Dr. Steven L. Stephenson, Department of Biology, Fairmont State College, Fairmont, WV; Dr. Donna Ford-Werntz, Eberly College of Arts and Sciences, West Virginia University, Morgantown, WV; Dr.