1 SI Units 2 Atomic Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

The Kibble Balance and the Kilogram
C. R. Physique 20 (2019) 55–63 Contents lists available at ScienceDirect Comptes Rendus Physique www.sciencedirect.com The new International System of Units / Le nouveau Système international d’unités The Kibble balance and the kilogram La balance de Kibble et le kilogramme ∗ Stephan Schlamminger , Darine Haddad NIST, 100 Bureau Drive, Gaithersburg, MD 20899, USA a r t i c l e i n f o a b s t r a c t Article history: Dr. Bryan Kibble invented the watt balance in 1975 to improve the realization of the unit Available online 25 March 2019 for electrical current, the ampere. With the discovery of the Quantum Hall effect in 1980 by Dr. Klaus von Klitzing and in conjunction with the previously predicted Josephson effect, Keywords: this mechanical apparatus could be used to measure the Planck constant h. Following a Unit of mass proposal by Quinn, Mills, Williams, Taylor, and Mohr, the Kibble balance can be used to Kilogram Planck constant realize the unit of mass, the kilogram, by fixing the numerical value of Planck’s constant. Kibble balance In 2017, the watt balance was renamed to the Kibble balance to honor the inventor, who Revised SI passed in 2016. This article explains the Kibble balance, its role in the redefinition of the Josephson effect unit of mass, and attempts an outlook of the future. Quantum Hall effect Published by Elsevier Masson SAS on behalf of Académie des sciences. This is an open access article under the CC BY-NC-ND license Mots-clés : (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

A) B) C) D) 1. Which Is an SI Unit for Work Done on an Object? A) Kg•M/S
1. Which is an SI unit for work done on an object? 10. Which is an acceptable unit for impulse? A) B) A) N•m B) J/s C) J•s D) kg•m/s C) D) 11. Using dimensional analysis, show that the expression has the same units as acceleration. [Show all the 2. Which combination of fundamental units can be used to express energy? steps used to arrive at your answer.] A) kg•m/s B) kg•m2/s 12. Which quantity and unit are correctly paired? 2 2 2 C) kg•m/s D) kg•m /s A) 3. A joule is equivalent to a B) A) N•m B) N•s C) N/m D) N/s C) 4. A force of 1 newton is equivalent to 1 A) B) D) C) D) 13. Which two quantities are measured in the same units? 5. Which two quantities can be expressed using the same A) mechanical energy and heat units? B) energy and power A) energy and force C) momentum and work B) impulse and force D) work and power C) momentum and energy 14. Which is a derived unit? D) impulse and momentum A) meter B) second 6. Which pair of quantities can be expressed using the same C) kilogram D) Newton units? 15. Which combination of fundamental unit can be used to A) work and kinetic energy express the weight of an object? B) power and momentum A) kilogram/second C) impulse and potential energy B) kilogram•meter D) acceleration and weight C) kilogram•meter/second 7. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

(Mks) Electromagnetic Units (Ohm, Volt, Ampere, Farad, Coulomb
VOL. 21, 1935 ENGINEERING: A. E. KENNELLY 579 Valley, Ventura County, California. In the history of North American mammal life this fauna has a position immediately antecedent to that of the Titanotherium Zone. Above this stage in the Sespe is a considerable thickness of land-laid material and some of these deposits are without much doubt lower Oligocene in age although in the absence of fossil remains this cannot be definitely proved at the present time. 2 Gilbert, G. K., U. S. Geog. Surv. West 100th Mer., Wheeler Surv., 3, 33 (1875). 3 Turner, H. W., U. S. Geol. Surv. 21st Ann. Rpt., Pt. 2, 197-208 (1900). 4 Spurr, J. E., Jour. Geol., 8, 633 (1900). 6 Spurr, J. E., U. S. Geol. Surv. Bull., 208, 19, 185 (1903). 6 Spurr, J. E., U. S. Geol. Surv. Prof. Pap. 42, 51-70 (1905). 7Ball, S. H., U. S. Geol. Surv. Bull., 308, 32-34, 165-166 (1907). ADOPTION OF THE METER-KILOGRAM-MASS-SECOND (M.K.s.) ABSOLUTE SYSTEM OF PRACTICAL UNITS BY THE INTER- NATIONAL ELE CTRO TECHNICAL COMMISSION (I.E.C.), BR UXELLES, JUNE, 1935 By ARTHUR E. KENNELLY SCHOOL OF ENGINEERING, HARVARD UNIVERSITY Communicated August 9, 1935 At its plenary meeting in June, 1935, in Scheveningen-Bruxelles, the I.E.C. unanimously adopted the M.K.S. System of Giorgi, as a compre- hensive absolute practical system of scientific units. The last preceding international action of a similar character was in 1881, when the International Electrical Congress of Paris' adopted the centimeter-gram-second (C.G.S.) system. -
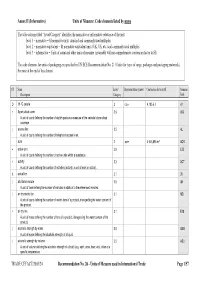
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

The New Definition of the Kilogram: Guide for Teachers and Students
The new definition of the kilogram: Guide for teachers and students On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition of Planck’s constant h=6.626 070 15 10-34 kg m2/s. This is a major change for metrology, but also a challenge for teachers to explain what now defines the unit of mass. However, this is also an opportunity to educate students and the public about modern science. Ideally, every high school teacher would tell his or her science class about this historic change. Here we provide a way of explaining the new kg in a direct and simple way. First, one should illustrate the concept of defining units through fundamental constants. Time is directly defined by the frequency of an atomic clock (more precisely, inside the cesium atom, there is an oscillation at νCs = 9,192,631,770 Hertz in the motion of the nuclear magnetic moment and electron magnetic moment). Length (or the meter) used to be defined by a 1 m long bar made of a platinum/iridium alloy. In 1960, the meter was redefined as 1,650,763.73 wavelengths of the orange- red light emitted by the Kr-86 isotope of the krypton atom. This was an important step from using imperfect man-made objects to perfect objects made by nature (cesium atoms, krypton atoms) which allows every country or laboratory to have a primary standard. Since time can be measured more accurately than length, the meter was redefined in 1984 by defining the numerical value of the speed of light as c= 299,792,458 meter/second. -

UNITS of WEIGHT and MEASURE International (Metric) and U.S
I \ ___^am UNITS OF WEIGHT AND MEASURE International (Metric) and U.S. Customary Definitions and Tables of Equivalents ivit I crv¥Hi\u M I I I Arm 'K^ he I I ^Nfck. r a law I I mmm I m mmJr \mw I mum lARE-ACRt STANDARDS U.S. DEPARTMENT OF COMMERCE / NATIONAL BUREAU OF Miscellaneous Publication 286 : THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assur- ing maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coor- dinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Phys- ics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics 2—Radio Standards Laboratory, 2 which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Reference Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of materials. -

Modernizing the SI – Implications of Recent Progress with the Fundamental Constants Nick Fletcher, Richard S
Modernizing the SI – implications of recent progress with the fundamental constants Nick Fletcher, Richard S. Davis, Michael Stock and Martin J.T. Milton, Bureau International des Poids et Mesures (BIPM), Pavillon de Breteuil, 92312 Sèvres CEDEX, France. e-mail : [email protected] Abstract Recent proposals to re-define some of the base units of the SI make use of definitions that refer to fixed numerical values of certain constants. We review these proposals in the context of the latest results of the least-squares adjustment of the fundamental constants and against the background of the difficulty experienced with communicating the changes. We show that the benefit of a definition of the kilogram made with respect to the atomic mass constant (mu) may now be significantly stronger than when the choice was first considered 10 years ago. Introduction The proposal to re-define four of the base units of the SI with respect to fixed numerical values of four constants has been the subject of much discussion and many publications. Although the possibility had been foreseen in publications during the 1990’s [1] they were not articulated as a complete set of proposals until 2006 [2]. Subsequently, the General Conference on Weights and Measures, the forum for decision making between the Member States of the BIPM on all matters of measurement science and measurement units, addressed the matter at its 23rd meeting in 2007. It recognised the importance of considering such a re- definition, and invited the NMIs to “come to a view on whether it is possible”. More recently at its 24th meeting in 2011, it noted that progress had been made towards such a re-definition and invited a final proposal when the experimental data were sufficiently robust to support one. -

Solutions to Problem Set # 5
ASTRONOMY 294Z: The History of the Universe Professor Barbara Ryden SOLUTIONS TO PROBLEM SET # 5 1) [20 points] Einstein showed that mass (M) and energy (E) are interchange- able: E = Mc2, where c is the speed of light. This implies, for instance, that 1 kilogram of matter is equivalent to an energy E = (1 kg)£(3£108 m=sec)2 = 9£1016 kg m2=sec2. An energy of 1 kg m2=sec2 is known as 1 joule, for short. The joule is not a unit of energy that is used much in everyday life. To give you a sense of scale, burning one gallon of gasoline releases 1:3 £ 108 joules (130 million joules) of energy. Okay, enough background. Here's the question: If you were capable of converting mass to energy with 100% e±ciency, how much mass M would you need to produce an energy E = 1:3 £ 108 joules? If E = Mc2, then M = E=c2. Thus, to produce E = 1:3 £ 108 joules = 1:3 £ 108 kg m2=sec2, the amount of mass required is E 1:3 £ 108 kg m2=sec2 M = = = 1:44 £ 10¡9 kg : (1) c2 (3 £ 108m=sec)2 This mass can also be written as 1.44 micrograms; it's roughly equivalent to the mass of a single grain of sand 0.1 millimeter across. 2) [20 points] The total annual energy consumption in the U.S.A. is 1020 joules. If all this energy were produced by burning gasoline, how many gallons of gasoline would be required in one year? Hoover Reservoir, just northeast of Columbus, has a capacity of 2:1 £ 1010 gallons.