UNITS of MEASURE Textbook Appendix A
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Metric System: America Measures Up. 1979 Edition. INSTITUTION Naval Education and Training Command, Washington, D.C
DOCONENT RESUME ED 191 707 031 '926 AUTHOR Andersonv.Glen: Gallagher, Paul TITLE The Metric System: America Measures Up. 1979 Edition. INSTITUTION Naval Education and Training Command, Washington, D.C. REPORT NO NAVEDTRA,.475-01-00-79 PUB CATE 1 79 NOTE 101p. .AVAILABLE FROM Superintendent of Documents, U.S. Government Printing .Office, Washington, DC 2040Z (Stock Number 0507-LP-4.75-0010; No prise quoted). E'DES PRICE MF01/PC05 Plus Postage. DESCRIPTORS Cartoons; Decimal Fractions: Mathematical Concepts; *Mathematic Education: Mathem'atics Instruction,: Mathematics Materials; *Measurement; *Metric System; Postsecondary Education; *Resource Materials; *Science Education; Student Attitudes: *Textbooks; Visual Aids' ABSTRACT This training manual is designed to introduce and assist naval personnel it the conversion from theEnglish system of measurement to the metric system of measurement. The bcokteliswhat the "move to metrics" is all,about, and details why the changeto the metric system is necessary. Individual chaPtersare devoted to how the metric system will affect the average person, how the five basic units of the system work, and additional informationon technical applications of metric measurement. The publication alsocontains conversion tables, a glcssary of metric system terms,andguides proper usage in spelling, punctuation, and pronunciation, of the language of the metric, system. (MP) ************************************.******i**************************** * Reproductions supplied by EDRS are the best thatcan be made * * from -

NO CALCULATORS! APES Summer Work: Basic Math Concepts Directions: Please Complete the Following to the Best of Your Ability
APES Summer Math Assignment 2015 ‐ 2016 Welcome to AP Environmental Science! There will be no assigned reading over the summer. However, on September 4th, you will be given an initial assessment that will check your understanding of basic math skills modeled on the problems you will find below. Topics include the use of scientific notation, basic multiplication and division, metric conversions, percent change, and dimensional analysis. The assessment will include approximately 20 questions and will be counted as a grade for the first marking period. On the AP environmental science exam, you will be required to use these basic skills without the use of a calculator. Therefore, please complete this packet without a calculator. If you require some review of these concepts, please go to the “About” section of the Google Classroom page where you will find links to videos that you may find helpful. A Chemistry review packet will be due on September 4th. You must register for the APES Summer Google Classroom page using the following code: rskj5f in order to access the assignment. The Chemistry review packet does not have to be completed over the summer but getting a head start on the assignment will lighten your workload the first week of school. A more detailed set of directions are posted on Google Classroom. NO CALCULATORS! APES Summer Work: Basic Math Concepts Directions: Please complete the following to the best of your ability. No calculators allowed! Please round to the nearest 10th as appropriate. 1. Convert the following numbers into scientific notation. 16, 502 = _____________________________________ 0.0067 = _____________________________________ 0.015 = _____________________________________ 600 = _____________________________________ 3950 = _____________________________________ 0.222 = _____________________________________ 2. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Chapter 5 Dimensional Analysis and Similarity
Chapter 5 Dimensional Analysis and Similarity Motivation. In this chapter we discuss the planning, presentation, and interpretation of experimental data. We shall try to convince you that such data are best presented in dimensionless form. Experiments which might result in tables of output, or even mul- tiple volumes of tables, might be reduced to a single set of curves—or even a single curve—when suitably nondimensionalized. The technique for doing this is dimensional analysis. Chapter 3 presented gross control-volume balances of mass, momentum, and en- ergy which led to estimates of global parameters: mass flow, force, torque, total heat transfer. Chapter 4 presented infinitesimal balances which led to the basic partial dif- ferential equations of fluid flow and some particular solutions. These two chapters cov- ered analytical techniques, which are limited to fairly simple geometries and well- defined boundary conditions. Probably one-third of fluid-flow problems can be attacked in this analytical or theoretical manner. The other two-thirds of all fluid problems are too complex, both geometrically and physically, to be solved analytically. They must be tested by experiment. Their behav- ior is reported as experimental data. Such data are much more useful if they are ex- pressed in compact, economic form. Graphs are especially useful, since tabulated data cannot be absorbed, nor can the trends and rates of change be observed, by most en- gineering eyes. These are the motivations for dimensional analysis. The technique is traditional in fluid mechanics and is useful in all engineering and physical sciences, with notable uses also seen in the biological and social sciences. -

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Scale on Paper Between Technique and Imagination. Example of Constant’S Drawing Hypothesis
S A J _ 2016 _ 8 _ original scientific article approval date 16 12 2016 UDK BROJEVI: 72.021.22 7.071.1 Констант COBISS.SR-ID 236416524 SCALE ON PAPER BETWEEN TECHNIQUE AND IMAGINATION. EXAMPLE OF CONSTANT’S DRAWING HYPOTHESIS A B S T R A C T The procedure of scaling is one of the elemental routines in architectural drawing. Along with paper as a fundamental drawing material, scale is the architectural convention that follows the emergence of drawing in architecture from the Renaissance. This analysis is questioning the scaling procedure through the position of drawing in the conception process. Current theoretical researches on architectural drawing are underlining the paradigm change that occurred as a sudden switch from handmade to computer generated drawing. This change consequently influenced notions of drawing materiality, relations to scale and geometry. Developing the argument of scaling as a dual action, technical and imaginative, Constant’s New Babylon drawing work is taken as an example to problematize the architect-project-object relational chain. Anđelka Bnin-Bninski KEY WORDS 322 Maja Dragišić University of Belgrade - Faculty of Architecture ARCHITECTURAL DRAWING SCALING PAPER DRAWING INHABITATION CONSTANT’S NEW BABYLON S A J _ 2016 _ 8 _ SCALE ON PAPER BETWEEN TECHNIQUE AND IMAGINATION. INTRODUCTION EXAMPLE OF CONSTANT’S DRAWING HYPOTHESIS This study intends to question the practice of scaling in architectural drawing as one of the elemental routines in the contemporary work of an architect. The problematization is built on the relation between an architect and the drawing process while examining the evolving role of drawing in the architectural profession. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

How Are Units of Measurement Related to One Another?
UNIT 1 Measurement How are Units of Measurement Related to One Another? I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot express it in numbers, your knowledge is of a meager and unsatisfactory kind... Lord Kelvin (1824-1907), developer of the absolute scale of temperature measurement Engage: Is Your Locker Big Enough for Your Lunch and Your Galoshes? A. Construct a list of ten units of measurement. Explain the numeric relationship among any three of the ten units you have listed. Before Studying this Unit After Studying this Unit Unit 1 Page 1 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. High School Chemistry: An Inquiry Approach 1. Use the measuring instrument provided to you by your teacher to measure your locker (or other rectangular three-dimensional object, if assigned) in meters. Table 1: Locker Measurements Measurement (in meters) Uncertainty in Measurement (in meters) Width Height Depth (optional) Area of Locker Door or Volume of Locker Show Your Work! Pool class data as instructed by your teacher. Table 2: Class Data Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Width Height Depth Area of Locker Door or Volume of Locker Unit 1 Page 2 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -
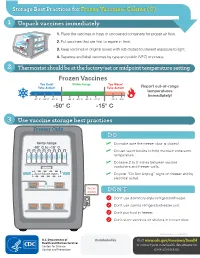
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -

The Kibble Balance and the Kilogram
C. R. Physique 20 (2019) 55–63 Contents lists available at ScienceDirect Comptes Rendus Physique www.sciencedirect.com The new International System of Units / Le nouveau Système international d’unités The Kibble balance and the kilogram La balance de Kibble et le kilogramme ∗ Stephan Schlamminger , Darine Haddad NIST, 100 Bureau Drive, Gaithersburg, MD 20899, USA a r t i c l e i n f o a b s t r a c t Article history: Dr. Bryan Kibble invented the watt balance in 1975 to improve the realization of the unit Available online 25 March 2019 for electrical current, the ampere. With the discovery of the Quantum Hall effect in 1980 by Dr. Klaus von Klitzing and in conjunction with the previously predicted Josephson effect, Keywords: this mechanical apparatus could be used to measure the Planck constant h. Following a Unit of mass proposal by Quinn, Mills, Williams, Taylor, and Mohr, the Kibble balance can be used to Kilogram Planck constant realize the unit of mass, the kilogram, by fixing the numerical value of Planck’s constant. Kibble balance In 2017, the watt balance was renamed to the Kibble balance to honor the inventor, who Revised SI passed in 2016. This article explains the Kibble balance, its role in the redefinition of the Josephson effect unit of mass, and attempts an outlook of the future. Quantum Hall effect Published by Elsevier Masson SAS on behalf of Académie des sciences. This is an open access article under the CC BY-NC-ND license Mots-clés : (http://creativecommons.org/licenses/by-nc-nd/4.0/). -
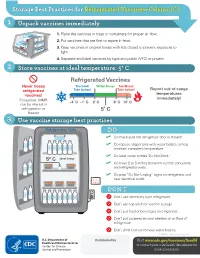
Storage Best Practices for Refrigerated Vaccines–Celsius (C)
Storage Best Practices for Refrigerated Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Store vaccines at ideal temperature: 5° C Refrigerated Vaccines Never freeze Too Cold! Within Range Too Warm! refrigerated Take Action! Take Action! Report out-of-range vaccines! temperatures Exception: MMR immediately! -4˚ C -1˚ C 2˚ C 8˚ C 10˚ C can be stored in refrigerator or 5˚ C freezer 3 Use vaccine storage best practices Refrigerator Only DO ✓ Do make sure the refrigerator door is closed! ✓ Do replace crisper bins with water bottles to help maintain consistent temperature. ✓ Do label water bottles "Do Not Drink." 5° C ideal temp ✓ Do leave 2 to 3 inches between vaccine containers and refrigerator walls. ✓ Do post “Do Not Unplug” signs on refrigerator and do not near electrical outlet. unplug DON’T ; Don’t use dormitory-style refrigerator. ; Don’t use top shelf for vaccine storage. ; Don’t put food or beverages in refrigerator. ; Don’t put vaccines on door shelves or on floor of refrigerator. Don’t drink from or remove water bottles. ; CS243541-C Revision February 2018 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Can you find at least 8 things that are wrong with vaccine storage in this refrigerator? 5° C ideal temp 2 When unpacking vaccines, why is it important to put the first-to-expire in the front? A.