Lymphatic Architecture of Suncus Murinus (House Musk Shrew) Palatum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of Greater Palatine Nerve Block with Intravenous Fentanyl for Postoperative Analgesia Following Palatoplasty in Children
Jemds.com Original Research Article Comparison of Greater Palatine Nerve Block with Intravenous Fentanyl for Postoperative Analgesia Following Palatoplasty in Children Amol Singam1, Saranya Rallabhandi2, Tapan Dhumey3 1Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha Maharashtra, India. 2Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha, Maharashtra, India. 3Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha, Maharashtra, India. ABSTRACT BACKGROUND Good pain relief after palatoplasty is important as inadequate analgesia with vigorous Corresponding Author: cry leads to wound dehiscence, removal of sutures and extra nursing care. Decrease Dr. Saranya Rallabhandi, in oxygen requirement and cardio-respiratory demand occur with good pain relief Assisstant Professor, and also promotes early recovery. Preoperative opioids have concerns like sedation, Department of Anesthesiology, AVBRH, DMIMS (DU), Sawangi Meghe, respiratory depression and airway compromise. Greater palatine nerve block with Wardha- 442001, Maharashtra, India. bupivacaine is safe and effective without the risk of respiratory depression. The study E-mail: [email protected] was done to compare pain relief postoperatively with intravenous fentanyl and greater palatine nerve block in children following palatoplasty. DOI: 10.14260/jemds/2020/549 METHODS How to Cite This Article: 80 children of ASA I & II, between 1 to 7 years were included and allocated into two Singam A, Rallabhandi S, Dhumey T. Comparison of greater palatine nerve block groups of 40 each. Analgesic medication was given preoperatively after induction of with intravenous fentanyl for postoperative general anaesthesia, children in Group B received greater palatine nerve block with analgesia following palatoplasty in -1 2 mL 0.25% inj. Bupivacaine (1 mL on each side) and Group F received 2 μg Kg I.V. -

Suncus Lixus – Greater Dwarf Shrew
Suncus lixus – Greater Dwarf Shrew transformed landscapes. It occurs in a number of protected areas and can be locally common in suitable habitat, such as riverine woodland, sandveld and moist grasslands. There is no evidence to suggest a net population decline. However, we caution that molecular data, coupled with further field surveys to delimit Photograph distribution more accurately, are needed to determine whether the highveld grassland and subtropical wanted grasslands subpopulations comprise separate species. If so, both species will need to be reassessed as high rates of grassland habitat loss in both regions may qualify one or both species for a threatened status. Key interventions include protected area expansion of moist grassland and riverine woodland habitats, as well as providing incentives for landowners to sustain natural Regional Red List status (2016) Least Concern* vegetation around wetlands and keep livestock or wildlife at ecological carrying capacity. National Red List status (2004) Data Deficient Regional population effects: There is a disjunct Reasons for change Non-genuine change: distribution between populations in the assessment region Change in risk and the rest of its range. This species is also a poor tolerance disperser. Thus there is not suspected to be a significant Global Red List status (2008) Least Concern rescue effect. TOPS listing (NEMBA) None CITES listing None Distribution Throughout the global range of the Greater Dwarf Shrew Endemic No there are only a few scattered records (Skinner & *Watch-list Data Chimimba 2005). However, it is a widespread species that ranges through East Africa, Central Africa and southern As the colloquial name indicates, although this is Africa. -
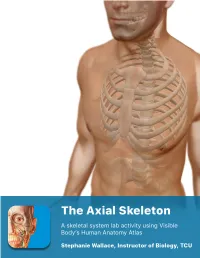
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Convergent Evolution of Olfactory and Thermoregulatory Capacities in Small Amphibious Mammals
Convergent evolution of olfactory and thermoregulatory capacities in small amphibious mammals Quentin Martineza,1, Julien Clavelb,c, Jacob A. Esselstynd,e, Anang S. Achmadif, Camille Grohég,h, Nelly Piroti,j, and Pierre-Henri Fabrea,k aInstitut des Sciences de l’Évolution de Montpellier (ISEM), CNRS, Institut de recherche pour le développement (IRD), Université de Montpellier (UM), UMR 5554, 34095 Montpellier, France; bDepartment of Life Sciences, The Natural History Museum, SW7 5DB London, United Kingdom; cUniv. Lyon Laboratoire d’Ecologie des Hydrosystèmes Naturels et Anthropisés, UMR CNRS 5023, Université Claude Bernard Lyon 1, École Nationale des Travaux Publics de l’État (ENTPE), F‐69622 Villeurbanne, Cedex, France; dMuseum of Natural Science, Louisiana State University, Baton Rouge, LA 70803; eDepartment of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803; fMuseum Zoologicum Bogoriense, Research Center for Biology, Indonesian Institute of Sciences (LIPI), 16911 Cibinong, Indonesia; gDivision of Paleontology, American Museum of Natural History, New York, NY 10024; hLaboratoire Paléontologie Évolution Paléoécosystèmes Paléoprimatologie (PALEVOPRIM, UMR 7262, CNRS-Institut écologie et environnement [INEE]), Université de Poitiers, 86073 Poitiers, Cedex 9, France; iInstitut de Recherche en Cancérologie de Montpellier (IRCM), INSERM, U1194 UM, Institut du Cancer de Montpellier (ICM), F-34298 Montpellier, Cedex 5, France; jRéseau d’Histologie Expérimentale de Montpellier, UMS3426 CNRS-US009 INSERM-UM, 34298 Montpellier, France; and kMammal Section, Department of Life Sciences, The Natural History Museum, SW7 5DB London, United Kingdom Edited by David B. Wake, University of California, Berkeley, CA, and approved February 28, 2020 (received for review October 11, 2019) Olfaction and thermoregulation are key functions for mammals. The partitioning has been documented in histological, airflow dynamic, former is critical to feeding, mating, and predator avoidance behaviors, and performance test studies (9–13). -

Morphometric Analysis of Greater Palatine Canal Via Cone-Beam Computed Tomography
DOI: 10.2478/bjdm-2018-0026 Y T E I C O S L BALKAN JOURNAL OF DENTAL MEDICINE A ISSN 2335-0245 IC G LO TO STOMA Morphometric Analysis of Greater Palatine Canal via Cone-Beam Computed Tomography SUMMARY Melih Özdede1, Elif Yıldızer Keriş2, Bülent Background/Aim: The morphology of the greater palatine canal (GPC) Altunkaynak3, İlkay Peker4 should be determined preoperatively to prevent possible complications in 1 Department of Dentomaxillofacial Radiology, surgical procedures required maxillary nerve block anesthesia and reduction Pamukkale University Faculty of Dentistry, of descending palatine artery bleeding. The purpose of this investigation was Denizli, Turkey to evaluate the GPC morphology. Material and Methods: In this retrospective 2 Canakkale Dental Hospital, Çanakkale, Turkey cross-sectional study, cone-beam computed tomography images obtained for 3 Department of Statistics, Gazi University various causes of 200 patients (females, 55%; males, 45%) age ranged between Faculty of Arts and Sciences, 18 and 86 (mean age±standard deviation=47±13.6) were examined. The mean Ankara, Turkey 4 Department of Dentomaxillofacial Radiology, length, mean angles of the GPC and anatomic routes of the GPC were evaluated. Gazi University Faculty of Dentistry, Results: The mean length of the GPC was found to be 31.07 mm and 32.01 mm Ankara, Turkey in sagittal and coronal sections, respectively. The mean angle of the GPC was measured as 156.16° and 169.23° in sagittal and coronal sections. The mean angle of the GPC with horizontal plane was measured as 113.76° in the sagittal sections and 92.94° in the coronal sections. -

Molecular Phylogenetics of Shrews (Mammalia: Soricidae) Reveal Timing of Transcontinental Colonizations
Molecular Phylogenetics and Evolution 44 (2007) 126–137 www.elsevier.com/locate/ympev Molecular phylogenetics of shrews (Mammalia: Soricidae) reveal timing of transcontinental colonizations Sylvain Dubey a,*, Nicolas Salamin a, Satoshi D. Ohdachi b, Patrick Barrie`re c, Peter Vogel a a Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland b Institute of Low Temperature Science, Hokkaido University, Sapporo 060-0819, Japan c Laboratoire Ecobio UMR 6553, CNRS, Universite´ de Rennes 1, Station Biologique, F-35380, Paimpont, France Received 4 July 2006; revised 8 November 2006; accepted 7 December 2006 Available online 19 December 2006 Abstract We sequenced 2167 base pairs (bp) of mitochondrial DNA cytochrome b and 16S, and 1390 bp of nuclear genes BRCA1 and ApoB in shrews taxa (Eulipotyphla, family Soricidae). The aim was to study the relationships at higher taxonomic levels within this family, and in particular the position of difficult clades such as Anourosorex and Myosorex. The data confirmed two monophyletic subfamilies, Soric- inae and Crocidurinae. In the former, the tribes Anourosoricini, Blarinini, Nectogalini, Notiosoricini, and Soricini were supported. The latter was formed by the tribes Myosoricini and Crocidurini. The genus Suncus appeared to be paraphyletic and included Sylvisorex.We further suggest a biogeographical hypothesis, which shows that North America was colonized by three independent lineages of Soricinae during middle Miocene. Our hypothesis is congruent with the first fossil records for these taxa. Using molecular dating, the first exchang- es between Africa and Eurasia occurred during the middle Miocene. The last one took place in the Late Miocene, with the dispersion of the genus Crocidura through the old world. -

Modeling the Jaw Mechanism of Pleuronichthys Verticalis: the Morphological Basis of Asymmetrical Jaw Movements in a Flatfish
JOURNAL OF MORPHOLOGY 256:1–12 (2003) Modeling the Jaw Mechanism of Pleuronichthys verticalis: The Morphological Basis of Asymmetrical Jaw Movements in a Flatfish Alice Coulter Gibb* Department of Ecology and Evolutionary Biology, University of California, Irvine, California ABSTRACT Several flatfish species exhibit the unusual water column for potential predators or prey. How- feature of bilateral asymmetry in prey capture kinemat- ever, the presence of both eyes on the same side of ics. One species, Pleuronichthys verticalis, produces lat- the head (i.e., the eyed side) also causes morpholog- eral flexion of the jaws during prey capture. This raises ical asymmetry of the skull and jaws (Yazdani, two questions: 1) How are asymmetrical movements gen- 1969). erated, and 2) How could this unusual jaw mechanism have evolved? In this study, specimens were dissected to Morphological asymmetry of the feeding appara- determine which cephalic structures might produce asym- tus creates the potential for another unusual verte- metrical jaw movements, hypotheses were formulated brate trait: asymmetry in jaw movements during about the specific function of these structures, physical prey capture. Two species of flatfish are known to models were built to test these hypotheses, and models exhibit asymmetrical jaw movements during prey were compared with prey capture kinematics to assess capture (Gibb, 1995, 1996), although the type of their accuracy. The results suggest that when the neuro- asymmetrical movement (i.e., kinematic asymme- cranium rotates dorsally the premaxillae slide off the try) is different in the two species examined. One smooth, rounded surface of the vomer (which is angled species, Xystreurys liolepis, produces limited kine- toward the blind, or eyeless, side) and are “launched” matic asymmetry during prey capture. -

Mandibular Jaw Movement and Masticatory Muscle Activity During Dynamic Trunk Exercise
dentistry journal Article Mandibular Jaw Movement and Masticatory Muscle Activity during Dynamic Trunk Exercise Daisuke Sugihara, Misao Kawara, Hiroshi Suzuki * , Takashi Asano, Akihiro Yasuda, Hiroki Takeuchi, Toshiyuki Nakayama, Toshikazu Kuroki and Osamu Komiyama Division of Oral Function and Rehabilitation, Department of Oral Health Science, Nihon University School of Dentistry at Matsudo, 870-1 Sakaecho, Nishi-2, Matsudo, Chiba 271-8587, Japan; [email protected] (D.S.); [email protected] (M.K.); [email protected] (T.A.); [email protected] (A.Y.); [email protected] (H.T.); [email protected] (T.N.); [email protected] (T.K.); [email protected] (O.K.) * Correspondence: [email protected]; Tel.: +81-47-360-9642 Received: 16 October 2020; Accepted: 30 November 2020; Published: 2 December 2020 Abstract: The examination of jaw movement during exercise is essential for an improved understanding of jaw function. Currently, there is no unified view of the mechanism by which the mandible is fixed during physical exercise. We hypothesized that during strong skeletal muscle force exertion in dynamic exercises, the mandible is displaced to a position other than the maximal intercuspal position and that mouth-opening and mouth-closing muscles simultaneously contract to fix the displaced mandible. Therefore, we simultaneously recorded mandibular jaw movements and masticatory muscle activities during dynamic trunk muscle force exertion (deadlift exercise) in 24 healthy adult males (age, 27.3 2.58 years). The deadlift was divided into three steps: ± Ready (reference), Pull, and Down. -

Pristipomoides Auricilla (Jordan, Evermann, and Tanaka, 1927) (Plate X, 67) Frequent Synonyms / Misidentifications: None / Other Species of Pristipomoides
click for previous page Perciformes: Percoidei: Lutjanidae 2909 Pristipomoides auricilla (Jordan, Evermann, and Tanaka, 1927) (Plate X, 67) Frequent synonyms / misidentifications: None / Other species of Pristipomoides. FAO names: En - Goldflag jobfish; Fr - Colas drapeau; Sp - Panchito abanderado. Diagnostic characters: Body elongate, laterally compressed. Nostrils on each side of snout close together. Jaws about equal or lower jaw protruding slightly. Premaxillae protrusible. Maxilla extending to vertical through anterior part of eye or slightly beyond. Upper and lower jaws both with an outer row of conical and canine teeth and an inner band of villiform teeth; vomer and palatines with teeth, those on vomer in triangular patch; no teeth on tongue. Maxilla without scales or longitudinal ridges. Interorbital region flattened. First gill arch with 8 to 11 gill rakers on upper limb, 17 to 21 on lower limb (total 27 to 32). Dorsal fin continuous, not deeply incised near junction of spinous and soft portions. Last soft ray of both dorsal and anal fins well produced, longer than next to last ray. Caudal fin forked. Pectoral fins long, equal to or somewhat shorter than head length. Dorsal fin with X spines and 11 soft rays. Anal fin with III spines and 8 soft rays. Pectoral-fin rays 15 or 16. Membranes of dorsal and anal fins without scales. Tubed lateral-line scales 67 to 74. Colour: body purplish or brownish violet; sides with numerous yellow spots or faint yellow chevron-shaped bands; dorsal fin yellowish to yellowish brown; upper lobe of caudal fin yellow. Sexual dichromatism: males over 27 cm (fork length) with much yellow on lower lobe of caudal fin, usually forming a distinct blotch; females with or without yellowish colour on lower lobe of caudal fin, but if yellow present, not forming a distinct blotch. -

Talpa Europaea), Captured in Central Poland in August 2013
www.nature.com/scientificreports OPEN Isolation and partial characterization of a highly divergent lineage of hantavirus Received: 25 October 2015 Accepted: 18 January 2016 from the European mole (Talpa Published: 19 February 2016 europaea) Se Hun Gu1, Mukesh Kumar1, Beata Sikorska2, Janusz Hejduk3, Janusz Markowski3, Marcin Markowski4, Paweł P. Liberski2 & Richard Yanagihara1 Genetically distinct hantaviruses have been identified in five species of fossorial moles (order Eulipotyphla, family Talpidae) from Eurasia and North America. Here, we report the isolation and partial characterization of a highly divergent hantavirus, named Nova virus (NVAV), from lung tissue of a European mole (Talpa europaea), captured in central Poland in August 2013. Typical hantavirus-like particles, measuring 80–120 nm in diameter, were found in NVAV-infected Vero E6 cells by transmission electron microscopy. Whole-genome sequences of the isolate, designated NVAV strain Te34, were identical to that amplified from the original lung tissue, and phylogenetic analysis of the full-length L, M and S segments, using maximum-likelihood and Bayesian methods, showed that NVAV was most closely related to hantaviruses harbored by insectivorous bats, consistent with an ancient evolutionary origin. Infant Swiss Webster mice, inoculated with NVAV by the intraperitoneal route, developed weight loss and hyperactivity, beginning at 16 days, followed by hind-limb paralysis and death. High NVAV RNA copies were detected in lung, liver, kidney, spleen and brain by quantitative real-time RT-PCR. Neuropathological examination showed astrocytic and microglial activation and neuronal loss. The first mole-borne hantavirus isolate will facilitate long-overdue studies on its infectivity and pathogenic potential in humans. -

Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” Is the Root Cause of Nearly All Malocclusions
Timothy Gross, DMD Part One In the Physiologic Orthodontics class at the Las Vegas Institute, we focus heavily on the “Big 3.” Orthodontic treatment and post treatment orthodontic stability necessitate management and correction of the “Big 3.” So, what is the “Big 3?” 1 2 3 Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” is the root cause of nearly all malocclusions. That’s right. Class I, Class II, and Class III malocclusions, crowding, deficient midfaces, cross bites, impacted teeth, high palatal arches, deep bites, open bites (and the list goes on) can all be explained by Enlow in his textbook, “The Essentials of Facial Growth.” That textbook is paramount for understanding normal facial growth and development and should be required reading for every dentist and dental specialist. Malocclusions, so predominant in our society, cannot be explained by genetics alone. Our phenotype, i.e. our genome in addition to environmental influences, is the result of corruption of the “Big 3.” The inability to breathe nasally, habitual mouth straight and make the upper and lower teeth couple breathing, and/or tongue habits lead to altered nicely. Unfortunately, the result is a skeletal and facial growth patterns which in turn lead to physiologic malocclusion with straight teeth, at observable dental and orthognathic malocclusions. the expense of the airway, the temporomandibular Orthodontic treatment is utilized to correct these joints and facial aesthetics. malocclusions, but the underlying pathophysiology must be addressed in order to achieve an optimal A Case Study… Me physiologic occlusion, balanced facial growth and As a 48 year old male, I had multiple issues: a facial beauty. -

A New Origin for the Maxillary Jaw
Developmental Biology 276 (2004) 207–224 www.elsevier.com/locate/ydbio A new origin for the maxillary jaw Sang-Hwy Leea, Olivier Be´dardb,1, Marcela Buchtova´b, Katherine Fub, Joy M. Richmanb,* aDepartment of Oral, Maxillofacial Surgery and Oral Science Research Center, Medical Science and Engineering Research Center, BK 21 Project for Medical Science, College of Dentistry Yonsei University, Seoul, Korea bDepartment of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada, V6T 1Z3 Received for publication 7 April 2004, revised 5 August 2004, accepted 31 August 2004 Available online 5 October 2004 Abstract One conserved feature of craniofacial development is that the first pharyngeal arch has two components, the maxillary and mandibular, which then form the upper and lower jaws, respectively. However, until now, there have been no tests of whether the maxillary cells originate entirely within the first pharyngeal arch or whether they originate in a separate condensation, cranial to the first arch. We therefore constructed a fate map of the pharyngeal arches and environs with a series of dye injections into stage 13–17 chicken embryos. We found that from the earliest stage examined, the major contribution to the maxillary bud is from post-optic mesenchyme with a relatively minor contribution from the maxillo-mandibular cleft. Cells labeled within the first pharyngeal arch contributed exclusively to the mandibular prominence. Gene expression data showed that there were different molecular codes for the cranial and caudal maxillary prominence. Two of the genes examined, Rarb (retinoic acid receptor b) and Bmp4 (bone morphogenetic protein) were expressed in the post-optic mesenchyme and epithelium prior to formation of the maxillary prominence and then were restricted to the cranial half of the maxillary prominence.