REVORD-DISSERTATION-2019.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nut Trees - 2021
NUT TREES - 2021 CHESTNUT – Chinese Castanea mollissima ‘Improved’ (Seed grown) #3 #7 pot A selection from a superior orchard strain with unusual vigor, uniformity, disease-resistance and superior nut production. At least two seedlings or cultivars must be planted within 100 to 200 feet of each other to ensure cross-pollination and optimal fruit set. Seedlings commonly bear fruit in four to seven years. Mature trees may yield 35 to 55 pounds of nuts each year. Grows 30- 60 feet tall and 25 feet wide. PECAN Carya illinoinensis Pollinate with another variety of pecan for bigger crops. Grows 50' tall or more. ‘Kanza’ (Grafted) #3 pot Quality nuts that ripen before other varieties. Superior cold hardiness, disease resistant. 52% kernel. Grows 70' tall, 40-75' wide. ‘Lakota’ (Grafted) #3 pot High-quality nuts shell easily yielding cream-to-golden kernels with rich flavor. Early maturing. Strong, wind-resistant tree, disease-resistant to pecan scab. Ripens in mid- to late-October. ‘Peruque’ (Grafted) #3 pot Early season producer derived from a selection of native seedlings near the Mississippi River. Average production is 81 nuts per pound, with 59% kernel. Kernels are golden, with tight dorsal grooves and a deep basal cleft. Grows 70' tall, 40-75' wide. Native. AMERICAN FILBERT #3 pot Corylus americana Native shrub, excellent for naturalizing, woodland gardens and shade areas. Showy male flowers (catkins) add early spring interest, dark green leaves turn a beautiful kaleidoscope of colors in the fall. The nuts mature from September to October. Grows 8’ x 8’ forming a thicket. WALNUT Black Walnut – Juglans nigra #15 Native tree that provides excellent shade for large properties. -

RECOMMENDED SMALL TREES for CITY USE (Less Than 30 Feet)
RECOMMENDED SMALL TREES FOR CITY USE (Less than 30 feet) Scientific Name Common Name Comments Amelanchier arborea Serviceberry, shadbush, Juneberry Very early white flowers. Good for pollinators and wildlife. Amorpha fruticosa False indigobush Can form clusters. Legume. Good for pollinators. Asimina triloba Paw-paw Excellent edible fruit. Good for wildlife. Can be hard to establish. Chionanthus virginicus Fringe Tree Cornus mas Cornelian Cherry (Dogwood) Cornus spp. Shrub dogwoods – Gray, Pale, Can form clusters. Very good for wildlife Red-0sier, Alternate, Silky & pollinators. Corylus americana Hazelnut Very good for wildlife. Crataegus pruinosa Frost hawthorn Thorny, attractive white flowers. Good for wildlife. Hamamelis virginiana Witchhazel Good for pollinators Lindera benzoin Spicebush Very good for butterflies. Sweet-smelling aromatic leaves Malus spp. Crabapples – Iowa and Prairie Can get 25’ tall. Beautiful spring flowers. Good for wildlife. Oxydendrum arborum Sourwood Prunus americana Wild or American plum Can form clusters. Very good for wildlife. Can get 20’ tall. Prunus virginiana Chokecherry Can get 30’ tall. Good for wildlife and pollinators. Rhus aromatic Aromatic sumac Attractive to bees and butterflies. Sambucus canadensis Elderberry Edible black berries – good for wildlife and pollinators. Viburnum spp. Arrowwood, nannyberry, Early white flower clusters, very good for blackhaw wildlife. NOTES: • All the above small trees/shrubs prefer moist soil. Some, like the false Indigobush, silky and red-osier dogwoods, spicebush, and elderberry, can tolerate wet soils. None do well on dry sandy or rocky soils. • All prefer at least 3 hours of sun per day, and flower better when they can get 6 hours or more per day. Spicebush can tolerate full shade, but flowers better with 3-6 hours of sun. -

Hazelnuts Resistant to Eastern Filbert Blight: Are We There Yet?
Hazelnuts Resistant to Eastern Filbert Blight: Are We There Yet? Thomas J. Molnar, Ph.D. Plant Biology and Pathology Dept. Rutgers University The American Chestnut Society Annual Meeting October 22, 2011 Nut Tree Breeding at Rutgers Based on the tremendous genetic improvements demonstrated in several previously underutilized turf species, Dr. C. Reed Funk strongly believed similar work could be done with nut trees Title of project started in 1996: Underutilized Perennial Food Crops Genetic Improvement Tom Molnar and Reed Funk Program Adelphia Research Farm August 2001 Nut Breeding at Rutgers Starting in 1996, species of interest included – black walnuts, Persian walnuts and heartnuts – pecans, hickories Pecan shade trial – chestnuts, Adelphia 2000 – almonds, – hazelnuts We built a germplasm collection of over 25,000 trees planted across five Rutgers research farms – Cream Ridge Fruit Research Farm (Cream Ridge, NJ) – Adelphia Research Farm (Freehold, NJ) Pecan shade trial Adelphia 2008 – HF1, HF2, HF3 (North Brunswick, NJ) Nut Breeding at Rutgers Goals – Identify species that show the greatest potential for New Jersey and Mid- Atlantic region – Develop breeding program to create superior well- adapted cultivars that reliably produce high- quality, high-value crops • while requiring reduced inputs of pesticides, fungicides, management, etc. Nut Breeding at Rutgers While most species showed great promise for substantial improvement, we had to narrow our focus to be most effective Hazelnuts stood out as the species where we could make significant contributions in a relatively short period of time – Major focus since 2000 Hazelnuts at Rutgers Why we chose to focus on hazelnuts: – success of initial plantings made in 1996/1997 with few pests and diseases – short generation time and small plant size (4 years from seed to seed) – wide genetic diversity and the ability to hybridize different species – ease of making controlled crosses – backlog of information and breeding advances – existing technologies and markets for nuts Hazelnuts: Corylus spp. -

A Call for Hazelnuts Revisited That You Send up to 100 Nuts from a Multitude of Wild Bushes in Your Region
A Call for Hazelnuts Revisited that you send up to 100 nuts from a multitude of wild bushes in your region. This harvest season is over, Last year, our collaborative hybrid hazelnut so please be on the lookout for hazelnuts to collect consortium, comprised of Rutgers University, Oregon next fall. Remember that wild hazelnut plants may State University, the University of Nebraska, Lincoln, be spotted more easily in the early spring, when their and the National Arbor Day Foundation, put out a catkins are bright yellow and shedding pollen, at a request to members of the NNGA and the Arbor Day time before the forest is full of green leaves. So, bring Foundation, for collections of the wild American along some flagging ribbon when you take any late hazelnut Corylus americana. The response, between winter or early spring hikes. The nuts you send will be 2010 and 2011, has been overwhelming. We have germinated the following spring in our greenhouses currently received over 95 samples from 68 different at Rutgers and the University of Nebraska, Lincoln. locations. We are well on our way to amassing wild Over subsequent years, these seedlings will be grown hazelnut germplasm from every state in the United in the field and evaluated for EFB resistance and States within the native range. We have even received other traits like nut yield, size, and kernel quality. We some northern samples from Canada! Attached is a will also characterize the collection using molecular map showing how many different sites we’ve obtained tools (like SSR markers, developed at Oregon State nuts from in each state, and the remaining states we University) to assess diversity and population structure. -

Native Plant Summary
Proposed City of Whitewater Landscape Policy Point Summary Plant Category Point Value Large Deciduous Tree 150 Small Deciduous Tree 60 Evergreen Tree 40 Shrub (deciduous or evergreen) 20 Perennial Planting 20 (for every 20 sq. ft. of bed) Please refer to City of Whitewater Landscape Guidelines for complete definitions of above plants and recommendations of the best plants for various planting sites. Please choose plants that are native to Wisconsin whenever possible. All plans that are native to Wisconsin will have additional point values awarded in calculations. Contact the Urban Forestry Commission if you need help determining appropriate selections. Proposed Summary of Native Plants for Wisconsin To Aid in Advising on Proposed Landscapes (from Landscaping with Native Plants of Wisconsin by Lynn M. Steiner) Native Deciduous Trees for Landscape Use Acer species (maples) Betula nigra (river birch) C. douglasii (black hawthorn) Carpinus caroliniana (blue beech) Carya ovata (Shagbark hickory) Celtis occidentalis (hackberry) Crataegus crus-galli (cockspur hawthorn) Fagus grandifolia (American beech) Fraxinus pennsylvanica (Green ash) Gleditsia triacanthos (Honey locust) Gymnocladus dioica (Kentucky coffee tree) Juglans nigra (Black walnut) Larix laricina (Tamarack) M. coronaria (American crab apple) Malus ioensis (Iowa crab apple) Morus rubra (red mulberry) Nyssa sylvatica (black gum) Ostrya virginiana (Ironwood, hop hornbeam) P. grandidentata (big-toothed aspen) P. deltoides (plains cottonwood) Platanus occidentalis (sycamore) Populus tremuloides (quaking aspen) Prunus serotina (Black cherry) Quercus alba (White oak) S. amygdaloides (peach-leaved willow) Salix nigra (black willow) Sorbus americana (American white ash) Tilia americana (basswood) U. rubra (red elm) Ulmus americana (American elm) Native Deciduous Shrubs and Small Trees for Landscape Use Alnus incana ssp. -

Landscaping Near Black Walnut Trees
Selecting juglone-tolerant plants Landscaping Near Black Walnut Trees Black walnut trees (Juglans nigra) can be very attractive in the home landscape when grown as shade trees, reaching a potential height of 100 feet. The walnuts they produce are a food source for squirrels, other wildlife and people as well. However, whether a black walnut tree already exists on your property or you are considering planting one, be aware that black walnuts produce juglone. This is a natural but toxic chemical they produce to reduce competition for resources from other plants. This natural self-defense mechanism can be harmful to nearby plants causing “walnut wilt.” Having a walnut tree in your landscape, however, certainly does not mean the landscape will be barren. Not all plants are sensitive to juglone. Many trees, vines, shrubs, ground covers, annuals and perennials will grow and even thrive in close proximity to a walnut tree. Production and Effect of Juglone Toxicity Juglone, which occurs in all parts of the black walnut tree, can affect other plants by several means: Stems Through root contact Leaves Through leakage or decay in the soil Through falling and decaying leaves When rain leaches and drips juglone from leaves Nuts and hulls and branches onto plants below. Juglone is most concentrated in the buds, nut hulls and All parts of the black walnut tree produce roots and, to a lesser degree, in leaves and stems. Plants toxic juglone to varying degrees. located beneath the canopy of walnut trees are most at risk. In general, the toxic zone around a mature walnut tree is within 50 to 60 feet of the trunk, but can extend to 80 feet. -
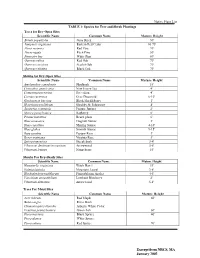
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees For
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees for Dry-Open Sites Scientific Name Common Name Mature Height Betula populifolia Gray Birch 30' Juniperis virginiana Eastern Red Cedar 10-75' Pinus resinosa Red Pine 70' Pin us rigida Pitch Pine 50' Pinus stro bus White Pine 80' Quercus rubra Red Oak 70' Quercus coccinea Scarlet Oak 70' Quercus velutina Black Oak 70' Shrubs for Dry-Open Sites Scientific Name 'Common Name Mature Height Amelanchier canadensis Shadbush 15' Ceanothus americanus New Jersey Tea 4' Comptonia peregrina Sweetfern 4' Cornus racemosa Gray Dogwood 6-10' Gaylussacia baccata Black Huckleberry l' Hypericum prolificum Shrubby St. Johnswort 4' Juniperus communis Pasture Juniper 2' Myrica pensylvanica Bayberry 6' Prunus maritima Beach plum 6' Rhus aromatica Fragrant Sumac 3' Rhus copallina Shining Sumac 4-10' Rhus glabra Smooth Sumac 9-15' Rosa carolina Pasture Rose 3' Rosa virginiana Virginia Rose 3' Spirea tomentosa Steeplebush 3-4' Viburnum dentatum/recognitum Arrowwood 5-8' Viburnum lentago Nannyberry 15' Shrubs For Dry-Shady Sites Scientific Name Common Name Mature Height Hamamelis wrginiana Witch Hazel 15' Kalmia latifolia Mountain Laurel 3-8' Rhododendron nudiflorum Pinxterbloom Azalea 4-6' Vaccinium angustifolium Lowbush Blueberry 2' Viburnum dentatum Arrowwood 5-8' Trees For Moist Sites Scientific Name Common Name Mature Height Acer rubrum Red Maple 60' Betula nigra River Birch Chamaecyparis thyoides Atlantic White Cedar Fraxinus pennsylvanica Green Ash 60' Picea mariana Black Spruce 40' Picea -

Oberlin's Experimental Hazelnut Orchard: Exploring Woody Agriculture’S Potential for Climate Change Mitigation and Food System Resilience
Oberlin's Experimental Hazelnut Orchard: Exploring Woody Agriculture’s Potential for Climate Change Mitigation and Food System Resilience By: Naomi Fireman Table of Contents Chapter 1: Introduction 3 1.1 Contextualizing the issue 3 1.2 Woody agriculture as a solution 5 1.3 Hazelnuts, Hybrid Hazelnuts, and Neohybrid Hazelnuts 9 Chapter 2: Biological Background, Fertilization, and Soils 11 Chapter 3: Methods 17 3.1 System Description 17 Management 18 3.2 Initial site conditions, preparation, and planting of trees 18 3.3 Current conditions 19 3.4 Independent and dependent variables 20 3.5 General tree care 20 Data Collection 21 3.6a Harvesting nut/husk clusters 21 3.6b Determining nut and husk biomass 22 3.7a Estimating biomass of woody stems 22 3.7c Developing allometric equations 25 3.8a Estimating leaf biomass 26 3.9 Measuring soil organic matter (SOM) 26 3.10 Statistical analysis 27 Chapter 4: Results 28 4.1 Analysis of covariance: effects of time and fertilizer treatment 28 4.1a Effects of time 28 4.1b Effects of fertilizer 29 4.1c Time and fertilizer treatment interaction 30 4.2 Correlations among variables 32 Chapter 5: Analysis & Discussion 33 5.1 Has the annual allocation of carbon to leaves, woody tissue, and nuts changed over time as the trees have matured? 33 5.2 How much carbon is being stored in the hazelnut system, where is it being stored, and how has this changed over time? 34 5.3 Is fertilization affecting patterns of carbon allocation and long-term storage? 35 5.4 Are these genetically diverse trees capable of producing -

Hazelnut Corylus Americana Kingdom: Plantae FEATURES Division: Magnoliophyta Hazelnut Is Also Known As American filbert
hazelnut Corylus americana Kingdom: Plantae FEATURES Division: Magnoliophyta Hazelnut is also known as American filbert. This Class: Magnoliopsida perennial shrub has leaves arranged alternately Order: Fagales along the twigs. The simple, double-toothed leaves are oval in shape. Leaves turn yellow, orange and red Family: Corylaceae in the fall of the year. The gray twigs and leafstalks ILLINOIS STATUS are covered with hairs. Green male and female flowers are borne on the same plant. The male common, native catkins are one to four inches in length and appear © Mary Kay Solecki before the leaves begin to unfurl. Catkins persist on the tree through the winter. The female flowers lack petals. Clustered near the twig tips, the bright red, female flowers are one-fourth inch wide. The fruit is a nut. The bristly nut husk is open at one end. Fruiting begins at about age six years. BEHAVIORS The hazelnut may be found throughout Illinois. It grows in moist prairies, fence rows, edges, dry woods and thickets. Flowers are produced in March and April. The seeds, leaves, stems and buds of this plant are eaten by wildlife. Hazelnut grows in small, shrubby groups because it sends up new plants from the roots. The flowers are pollinated by the wind. leaves ILLINOIS RANGE leaves and fruit © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. © Tom Robbins Aquatic Habitats wet prairies and fens Woodland Habitats upland deciduous forests Prairie and Edge Habitats black soil prairie; edge; shrub prairie © Illinois Department of Natural Resources. -

APPROVED PLANT LIST for JURISDICTIONAL PROJECTS
CARLISLE CONSERVATION COMISSION - APPROVED PLANT LIST for JURISDICTIONAL PROJECTS UNDER FORESTED BANKS & WATER WETLAND DRIER WETLANDS WET, SUNNY WATER'S (>1' INDICATOR TYPE BOTANICAL NAME COMMON NAME UPLAND & SHADE MEADOWS EDGE DEEP) STATUS Deciduous Trees Acer rubrum Red Maple x x x x FAC Acer saccharinum Silver Maple x x x x FACW Benthamidia (Cornus) florida Flowering Dogwood x x x FACU Betula nigra River Birch x x x x FACW Betula papyrifera Paper Birch x FACU Hamamelis virginiana Witch-Hazel x x FAC- Nyssa sylvatica Sweet Gum x x x x FAC Quercus alba White Oak x x x FACU Quercus rubra Red Oak x x x FACU- Evergreen Trees Juniperus virginiana Eastern Red Cedar x x UPL Pinus strobus White Pine x x x FACU Tsuga canadensis Eastern Hemlock x x FACU Evergreen Shrubs Juniperus horizontalis Prostrate Juniper x x FACU Kalmia latifolia Mountain Laurel x x x FACU Shrubs Amelanchier canadensis ShadBush x x x FAC Cephalanthus occidentalis Buttonbush x x x x x OBL Clethra alnifolia Sweet Pepper Bush x x x x x FAC+ Comptonia peregrina Sweet Fern x x UPL Corylus americana American Hazelnut x x x x FACU- Ilex verticillata Winterberry Holly x x x x FACW+ Lindera benzoin Spice Bush x x x FACW- Photinia (Aronia) arbutifolia Red Chokeberry x x FACW Photinia (Aronia) melanocarpa Black Chokeberry x x FAC Rhododendron viscosum Swamp Azalea x x x OBL Swida (Cornus) racemosa Grey Dogwood x x x x FAC Swida (Cornus) sericea Red Osier Dogwood x x x x FACW+ Vaccinium angustifolium Low Bush Blueberry x x x FACU- Vaccinium corymbosum High Bush Blueberry x x x x FACW -

Uncultivated Nuts of the United States
Uncultivated Nuts of Forest Service The United States Agriculture Information Bulletin Number 450 Arnold Krochmal Connie Krochmal Pî f3 3 i-O CO D OJ U TJ 1 ■> li> en 0 h- < 0 CM c 0 o £ Q -ce ■y^ CCÎ CD .si <r CO T— ■¿i a _,i 0 CO < 0 0» D 2: I— HJ Figure 1.—Cherokee woman pounding nuts with wooden mortar and pestle (courtesy of the Smithsonian Institution, Neg. No. 55, 445). Uncultivated Nuts of The United States by Arnold Krochmal, Economic Botanist, Southeastern Forest Experiment Station, Asheville, N.C., and Connie Krochmal, Volunteer Writer United States Department of Agriculture Forest Service june 1982 Library of Congress Catalog Card Number: 81-600067 Krochmal, Arnold; Krochmal, Connie. Uncultivated nuts of the United States. Agrie. Info. Bull. 450. Washington, DC : U.S. Department of Agri- culture; 1982.89 p. A handbook of nuts and nutlike seeds from trees and shrubs that are not cultivated in orchards but generally grow wild or that are sometimes grown as ornamentals. Descriptions, illustrations, and range maps help the reader to identify species. The nuts and seeds are also discussed as a renewable resource of present or potential use to human beings. KEYWORDS: Nutrition, renewable resources. Acknowledgments Study materials and samples were provided by David Stoller of the Cornell Plantations and Harold Grelen, Lisle Green, Franklin Bonner, Rudolph Strothmann, Douglas Roy, and David Priester, all of the Forest Service. Herbarium specimens and information on distribution and nomenclature were made available by Frederick Meyer and Peter Mazzeo, U.S. National Arboretum; Stanwyn Shetler, Smithsonian Institution; Carol Keener, Pennsylvania State University; Gene Wofford, University of Tennessee; Daniel Ward, University of Florida; John Freeman, Auburn University; L. -

(Castanea Dentata)-Dominated Community in Western Wisconsin
Mycorrhiza (2008) 19:27–36 DOI 10.1007/s00572-008-0200-7 ORIGINAL PAPER Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in Western Wisconsin Jonathan M. Palmer & Daniel L. Lindner & Thomas J. Volk Received: 13 June 2008 /Accepted: 28 August 2008 /Published online: 21 September 2008 # Springer-Verlag 2008 Abstract Circa 1900, a farmer from the eastern US planted ground molecular-based surveys and provides preliminary 11 American chestnut (Castanea dentata) seeds on a newly identification of ectomycorrhizal fungi associated with a established farm near West Salem in western Wisconsin. disjunct stand of American chestnut. These trees were very successful, producing a large stand of over 6,000 trees. Since this area is well outside the natural Keywords American chestnut . Chromelosporium . range of chestnut, these trees remained free from chestnut Cryphonectria parasitica . Ectomycorrhizae . blight until 1987. In the West Salem stand, chestnuts are the Eastern hardwood forests dominant species of a mixed forest community, reminiscent of the chestnut–oak ecosystems of pre-1900 Appalachia. To identify putative mycorrhizal associates of chestnut in this Introduction unique forest, our approach was twofold: (1) an extensive fruiting body survey was conducted for four seasons that American chestnut (Castanea dentata) was the dominant yielded approximately 100 putative mycorrhizal species tree of the Appalachian mountain range and surrounding and (2) a belowground molecular approach was used to areas prior to the accidental introduction of the chestnut generate DNA sequences of the internal transcribed spacer blight fungus, Cryphonectria parasitica, before the early region from ectomycorrhizae. Unexpectedly, chestnut did 1900s. Chestnut blight affected over four billion trees in not appear to be the dominant underground ectomycorrhizal- their native range over a period of approximately 50 years forming plant species.