Fluoride in Taiz
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conflict Snapshot – Yemen
Funded by the European Union COVID-19 SNAPSHOT in December 2020 Yemen Conflict Introduction The ongoing conflict in Yemen is complex and multi-layered, with the involvement of rival regional powers adding a geopolitical dimension to the war. As the national framework has disintegrated, local rivalries have intensified, leaving more room for foreign state interference in the country.1 Since March 2015, a dangerous combination of factors, driven by conflict and economic decline, and now exacerbated by COVID-19, have compounded the dire situation for over 24 million Yemenis, as reported by the UN, international non-governmental organisations (INGOs), and local sources. The country has been de facto split into two since several years: the North, controlled by the de-facto authorities of Ansarullah “Al-Houthi”, and the South, which is controlled by the internationally-recognised Hadi government (IRG). Data collection and analysis Data for this first COVID-19 snapshot report in Yemen Conflict was gathered from 31 October to 10 November 2020 in the following target zones: Tuban and Toor Al-Baha districts in Lahj governorate (located in the South, under IRG control), and Al-Mawasit and Jabal Habashi districts in Taiz governorate (located in the North but under IRG control). This report was prepared by an independent consultant and the Search for Common Ground (Search) Yemen team, with T support from Search’s COVID-19 Response Programming Team and Institutional Learning Team. For more information on the data collection methodology and tools used by Search for Common Ground for this research, click here. To cite this report: Search for Common Ground, COVID-19 Snapshot in Yemen Conflict, December 2020. -

How the Ongoing Crisis in Taiz Governorate Continues to Put Civilians at Risk
A crisis with no end in sight How the ongoing crisis in Taiz Governorate continues to put civilians at risk www.oxfam.org OXFAM BRIEFING NOTE – DECEMBER 2020 Despite a UN-brokered peace agreement in December 2018, the conflict in Yemen has run into its sixth year. In Taiz Governorate, civilians continue to bear the brunt of conflict. Every day, they face death or injury from indiscriminate attacks, gender- based violence in their homes and poor access to food, water and medical care. As people’s resources are further exhausted, their safety, security and well-being are only likely to worsen. The COVID-19 pandemic has added an additional layer to the ongoing crisis. The people of Taiz – and across Yemen as a whole – desperately need a lasting and inclusive peace process to end the world’s worst humanitarian crisis. © Oxfam International December 2020 This paper was written by Abdulwasea Mohammed, with support from Hannah Cooper. Oxfam acknowledges the assistance of Amr Mohammed, Georges Ghali, Helen Bunting, Martin Butcher, Nabeel Alkhaiaty, Omar Algunaid, Marina Di Lauro, Ricardo Fal-Dutra Santos, Ruth James and Tom Fuller in its production. Particular thanks go to the organizations and individuals that Oxfam spoke with as part of the research for this briefing note. It is part of a series of papers written to inform public debate on development and humanitarian policy issues. For further information on the issues raised in this paper please email [email protected] This publication is copyright but the text may be used free of charge for the purposes of advocacy, campaigning, education, and research, provided that the source is acknowledged in full. -

And the Qatari Dreams 00 Starts from Al-Hajriya of Taiz
Cover Title The Islah Party , The Yemen Vatican 00 (The Brothers') Rule inside The State !!! And the Qatari Dreams 00 Starts from Al-Hajriya of Taiz 1 The Title of the Report: Haq Organization's report : Taiz Governorate outside the state control . Al-Islah Party changed it into brother Emirate and into non-organized camps. For the armed bodies and terrorist's organizations funded by Qatar. 2 Field report Taiz Governorate Taiz Governorate outside the sovereignty of the state. The Content of the Report The content page No. Acknowledgment 4 Background 5 Taiz Governorate outside the sovereignty of 7 the state Destroying the last strongholds of state 20 Irregular training camps 32 Serious crimes according to Intel Law 47 Recommendations 64 Efforts and activities of the organization and access to international forums: The organization has made efforts and activities for 10 years, through which it has been keen on continuous work in monitoring the human rights situation, documenting it in accordance with international standards, preparing reports and publishing them before various media outlets and public opinion, and delivering them to the concerned authorities locally and internationally. We review the efforts of the organization through its field work in monitoring and following up the reality of human rights in Taiz Governorate five years ago, as the organization produced a number of field reports related to violations and serious crimes against human rights in Taiz governorate, namely: 1. The reality of human rights and informal prisons (October 2017) 2. Legitimate prisons and illegal prisons (January 2019) 3. The dangers of sectarianism and storming the old city ...... -

Parasitological and Biochemical Studies on Cutaneous Leishmaniasis in Shara'b District, Taiz, Yemen
Asmaa et al. Ann Clin Microbiol Antimicrob (2017) 16:47 DOI 10.1186/s12941-017-0224-y Annals of Clinical Microbiology and Antimicrobials RESEARCH Open Access Parasitological and biochemical studies on cutaneous leishmaniasis in Shara’b District, Taiz, Yemen Qhtan Asmaa1, Salwa AL‑Shamerii2, Mohammed Al‑Tag3, Adam AL‑Shamerii4, Yiping Li1* and Bashir H. Osman5 Abstract Background: The leishmaniasis is a group of diseases caused by intracellular haemofagellate protozoan parasites of the genus Leishmania. Leishmaniasis has diverse clinical manifestations; cutaneous leishmaniasis (CL) is the most com‑ mon form of leishmaniasis which is responsible for 60% of disability-adjusted life years. CL is endemic in Yemen. In Shara’b there is no reference study available to identify the prevalence of endemic diseases and no investigation has been conducted for diagnosing the diseases. Methods: This study was conducted in villages for CL which collected randomly. The study aimed at investigating the epidemiological factors of CL in Shara’b by using questioner. Symptoms of lesions in patients sufering from CL, confrmed by laboratory tests, gave a new evidence of biochemical diagnosis in 525 villagers aged between 1 and 60 years old. Venous bloods were collected from 99 patients as well as from 51 control after an overnight fast. Results: The percentage prevalence of CL was found 18.8%. The prevalence rate of infection among males (19.3%) was higher than females (18.40%). Younger age group (1–15) had a higher prevalence rate (20.3%) than the other age groups. Furthermore, the population with no formal education had the higher rate of infection (61% of the total). -
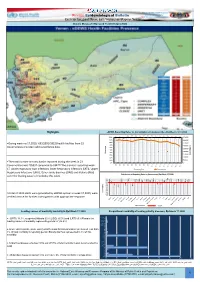
Eiectronic Integrated Disease Early Warning and Response System Volume 08,Lssue17,Epi Week 17,(20-26 April,2020)
Ministary Of Public Health Papulation Epidemiological Bulletin Primary Heath Care Sector Weekly DG for Diseases Control & Surveillance Eiectronic Integrated Disease Early Warning and Response System Volume 08,lssue17,Epi week 17,(20-26 April,2020) Highlights eDEWS Reporting Rates vs Consultations in Govemorates,Epi Weeks 1-17,2020 % % % % % % % % % % % % % % % % 97 97 97 95 % 96 96 96 96 95 95 95 95 100% 94 450000 96 96 92 96 90% 400000 •During week no.17,2020, %92(1991/1822) health facilites from 23 80% 350000 70% 300000 Governorates provided valid surveillance data. 60% 250000 50% 200000 Percentage 40% 150000 Consulttaions 30% 20% 100000 10% 50000 •The total number of consultation reported during the week in 23 0% 0 Wk 2 Wk Wk 1 Wk 3 Wk 4 Wk 5 Wk 6 Wk 7 Wk 8 Wk 9 Wk Wk 16 Wk Wk 11 Wk 12 Wk 13 Wk 14 Wk 15 Wk 17 Wk Governorates was 295637 compared to 334727 the previous reporting week 10 Wk 17. Acute respiratory tract infections lower Respiratory Infections (LRTI), Upper Reporting Rate Consultations Respiratory Infections (URTI), Other acute diarrhea (OAD) and Malaria (Mal) Distribution of Reporting Rates by Governoraes (Epi-Week 17,2020) were the leading cause of morbidity this week. % % % % % 100% % % % % % % % 95 % % % 97 98 100 90% 100 99 90 81 100 97 100 100 % 98 96 96 % % 80% % 88 % % 92 86 73 70% % % 77 74 60% 76 69 50% 40% 30% No. HF Reports 20% A total of 1332 alerts were generated by eDEWS system in week 17,2020, were 10% verified as true for further investigations with appropriate response 0% Reporting Rate Target Leading causes -

Resolving Displacement Essential for Long- Term Peace and Stability
18 September 2014 Yemen Resolving displacement essential for long- term peace and stability Intermittent armed conflict and violence have mainly taken place on three fronts in Yemen in recent years. The Shia al-Houthi movement has led a violent insurgency in the north, there has been civil unrest in central and southern regions, and government forces have clashed in the south with militants associated with the Southern Separatist Movement and Ansar al-Sharia, an offshoot of al-Qaida in the Arabian Peninsula (AQAP). The fighting has caused ongoing and often repeated internal displace- ment, which has taken place in the context of Internally displaced boys living in Al Madab settlement, Hajja, Yemen, fetch water a growing socio-economic and humanitarian from a well while using a donkey to transport the water to their home 100km away. crisis. Political instability has left the govern- Photo: NRC/Nashon Tado, June 2013 ment struggling to provide basic services, con- tributing to increased needs among vulnerable groups. As of July 2014, there were 334,626 internally displaced people (IDPs) registered by the government’s Executive Unit and the UN Refugee Agency (UNHCR), most of whom had fled conflict and violence. Disasters including flash floods and drought have also caused displacement, but there are few reliable estimates of its scale. IDPs and their host communities face many protection risks, including threats to their physical security and a lack of access to adequate shelter and basic services. Food insecurity and malnutrition rates are also particularly high among displaced communities, and IDPs face a heightened risk of serious human rights abuses. -

Geological and Structural Evolution of the Sharab Area, Southwest Yemen Abdul-Hamid Malek, Fuad Bagash, Abdul-Aleam Ahmed Al-Qadhi
International Journal of Scientific & Engineering Research Volume 12, Issue 4, April-2021 InternationalISSN 2229-5518 Journal of Scientific & Engineering Research, Volume 8, Issue 1, January-2017 224 ISSN 2229-5518 Geological and Structural Evolution of the Sharab area, Southwest Yemen Abdul-Hamid Malek, Fuad Bagash, Abdul-Aleam Ahmed Al-Qadhi Abstract— This paper presents the first attempt to give insight background on the lithological units and their structural evolution in the Sharab area of Yemen, based on the field work, satellite image analysis and available secondary data sources. Accordingly, geological map is constructed. Lithologically, the Sharab area is occupied by a few thousand meters thick of Precambrian basement rocks overlain by thick Mesozoic marine sediments (limestone and shale) and clastic sediments (sandstone, siltstone, mudstone and conglomerate). The Precambrian basement and Mesozoic sediments are coated by huge amounts of Tertiary basalts intercalated with acidic and volcaniclastic strata and injected by several types of igneous dykes, sills and plugs. Structurally, the study area is affected by six phases of deformations (D1-D6) took place in three stages; during the Precambrian (D1: D4), through Mesozoic (D5) and in the Cenozoic (D6). D1 of the Precambrian is characterized by penetrative linear foliation (S1), lineation (L1) and isoclinal, intrafolial folds (F1), while D2, D3 and D4 are characterized by a series of major and minor anticline and syncline folds (F2), ductile left-lateral shear zones, and right lateral ductile-brittle shear zones respectively. D5 generated during Mesozoic is represented by the obviously observed folds in the Jurassic limestone. The latest phase of deformation (D6) in Cenozoic is characterized by horizontal extension and vertical thinning, which led to wide fragmentation by E-W, NE- SW, WNW-ESE and NW-SE conjugate system of high-angle brittle normal faults and several distinctive horsts and grabens. -

GWQ Annual Repot Years of 2013, 2014 & 2015
GWQ Annual Repot Years of 2013, 2014 & 2015 1 Introduction: Generations Without Qat is a youth-based organization which started as a youth initiative in 2007. The organization starts to work in a voluntary basis with the aim of being agents of changes in Taiz city advocating for social, political and developmental rights. On the year of 2010 GWQ was able to register formally and became officially a licensed NGO and no profitable that aims to support youth and women and enhance their abilities to be socially and politically recognized. In this report, GWQ tried to present the main achievements of its work during the years 2013, 2014, and 2015. The combination of these years comes from the humble contribution and track record of GWQ during these years. Area of Interventions during 2013, 2014 & 2015: 1- Youth/Girls Empowerment. 2- Civic Education 3- Good Governance Main Achievements Youth Dialogue Project description: This project was implemented by GWQ and funded by the (USAID), this project was involved in the conflict prevention and resolution, peace and security. As this project took place in Taiz and Ibb governorates in a duration from 18/02/2013 until 17/05/2013. Project's activities: 1- Peace building. 2 2- wise governance. 3- Accepting the other. 4- Supporting the transitional phase. 5- Training local NGOs and local authorities' offices. Youth For Peace Lovers of love and peace symphony play Project description: This project was implemented by GWQ and funded by American Embassy and the IOM which was on peace, accepting the other, women participants and took place in Sana'a, Ibb and Taiz governorates with a duration from 2012 until 2013. -

Yemen Events Log 3
Yemen Events Log 3 This is a publicly available events log to keep track of the latest coalition airstrikes on civilians or civilian infrastructure in Yemen, plus any other significant reports or events that are related. It is being updated daily a couple of dedicated independent activists who have a concern for the people of Yemen and a desire to see the end of this unfolding catastrophe. If you would like to help, please drop me a direct message on Twitter. @jamilahanan For current data, May 2018 onwards, see here: May 2017 - April 2018 https://docs.zoho.com/file/1g2al5ce282ae1ccc4ea7ac011b61edb74b21 This log contains events from November 2016 - April 2017. Previous events can be found here: August 2016 - October 2016 https://docs.zoho.com/file/qqptj5d51d260604b48f691fb33fba2641be6 Before August 2016 https://docs.zoho.com/file/qu3o1a39ece47dff44380a9a48fdc45489ddf April 2017 30th April Legalcenter for Rights and Developement - Airstrikes April 30th 2017 https://www.facebook.com/lcrdye/photos/a.551858951631141.1073741828.551288185021551/8 18304141653286/?type=3&theater 29th April What are the reasons for the US-Saudi aggression on #Yemen, which have became known to all countries of the #world? https://twitter.com/PrincessOfYmn/status/858258474173706240 Yemen – the New Graveyard Where Empires Come to Die https://twitter.com/ShakdamC/status/858209772050558976 Legalcenter for Rights and Developement - Airstrikes April 29th 2017 https://www.facebook.com/lcrdye/photos/a.551858951631141.1073741828.551288185021551/8 17825941701106/?type=3&theater 28th April Legalcenter for Rights and Developement - Airstrikes April 28th 2017 https://www.facebook.com/lcrdye/photos/a.551858951631141.1073741828.551288185021551/8 17307905086243/?type=3&theater Sen. Rand Paul: The U.S. -

Final Report of Local Community Initiatives in Water N
G S C P Ghayth Aqua - t e c h 2012 Teamwork leader ABDULKHALEQ ALGHABERI Final report of Local Community initiatives in Water Governance ALMALIKA village initiative to prevent transfer of groundwater in - Bani HUSHAISH District- Sana’a to other areas for Qat cultivation Table of Contents Page N0 1. Reconnaissance and Identification of five local initiatives 1.1 INTRUDUCTION 2 1.2 Methodology & Approach Description: 3 1.3 Detailed description of five initiatives 4 2. Selection of the local Initiative for detailed study as for TOR 5 3. Summary of Al- Malikah initiative Case Study 6 4. Details Report of Al- MalikahCase Study 10 5. References 22 6. Annex1,summary 0n the 5 selected initiatives. First Local community based initiative for Spring Water 23 Management and Conservation in AL-QOSHE’Y Village- Otuma District-Dhamar province Second Community based Spate Water Management Initiative Moazi 31 spate diversion structure Shibam district , Hadhramaut Governorate Third Local community initiative on Monitoring Groundwater in 37 ALMAAFER District Taiz Governorate Fourth Local Community based initiative on management and 44 conservation of harvesting water Kohl Village – Dhebain District - Amran Governorate Fifth Local Community based initiative to prevent selling of ground 49 water in ALMALIKAH village- BaniHushish District- Sana’a 1 Page 1. Reconnaissance and Identification of five local initiatives ; 1.1 Introduction: The concept of water governance refers to deciding on certain weighted and balanced intervention by joining political, social, economic and administrative systems in order to develop and manage water resources, and to deliver water services, at different levels of society” (Global Water Partnership, 2003). -

Nutrition Survey of Taiz Governorate 2016 Final Report
Republic of Yemen Ministry of Public Health and Population Taiz Governorate Health Office Nutrition Survey of Taiz Governorate 2016 May 2016 Final Report 1 Contents Page 1. Executive Summary 7 2.Taiz Context 9 3. Methodology 10 3.1 Setting 10 3.2 Study and sampling design 11 3.3 Sampling Procedure (The second stage) 12 3.4 Survey Population and Data Collection Process 13 3.5 Measurement Standardization and Quality Control 14 3.6 Data Entry and Analysis 15 4. Assessment Results 17 4.1 Household Characteristics 17 4.2 Food Security 21 4.3 Child Nutrition 25 4.3.1 Distribution of U5 children 25 4.3.2 Wasting (WHZ) Indicators 26 4.3.3 Oedema and WHZ Indicators 28 4.3.4 Underweight (WAZ) Indicators 29 4.3.5 Stunting (HAZ) Indicators 31 4.3.6 MUAC Indicator of Children 36 4.4 Child Morbidity and Immunization 41 4.5 Infant and Young Child Feeding (IYCF) Practices 42 4.6 Child Mortality 43 4.7 Mid-Upper Arm Circumference (MUAC) Measurement for 44 Women 4.8 Discussion and variable associations 45 5. References 48 2 3 Acronyms TC: Taiz City THL: Taiz Highland TLL: Taiz Lowland HHs: Households YER: Yemeni Rial WHZ: Weight-for-height-z score WAZ: Weight-for-age-z score HAZ: Height-for-age-z score MUAC: Mid Upper Arm Circumference GAM: Global Acute Malnutrition MAM: Moderate Acute Malnutrition SAM: Severe Acute Malnutrition 95% CI: 95% Confidence Interval FT: Fisher Test SD: Standard Deviation U5: Under Five U5MR: Under Five Mortality Rate CMR: Crude Mortality Rate MDGs: Millennium Development Goals SDGs: Sustainable Development Goals BF: Breastfeeding MDD: Minimum Dietary Diversity MMF: Minimum Meal Frequency MAD: Minimum Acceptable Diet IYCF: Infant and Young Child Feeding 4 List of Tables Table 1: Key indicators of the nutritional survey of Taiz Governorate Table 2. -

YEMEN Situation Report April 2018
YEMEN Situation Report April 2018 General Context KEY FIGURES Over the month, the violence continues to plague the war-ravaged country which has raised serious protection concerns for civilian lives and resulted in worsening the overall humanitarian Total population in need situation and the suffering of Yemeni people. In Ibb hub, the overall response has decreased compared to last month due to the new lengthy 5.4 million coordination requirements demanded by NAMCHA (National Authority for the Management and Total population with acute needs Coordination of Humanitarian Affairs and Disaster Recovery) in addition to the slow internal pro- cedures of Partners and the challenge Partners are encountering to deliver the response in multi- 2.6 million ple locations in this more restrictive environment . Cluster targeted population In the southern part of Taizz, the internal clashes that have taken place inside Taiz city (Al 3 million Mudhaffar, Al Qahirah and Part of Salh districts) have affected the security situation and resulted Population assisted in the suspension of some NGOs programs and presence in the city. It was observed that the vulnerability among host community have increased recently which led to hostility against IDPs 268,791 as the humanitarian programs are often focusing mainly on IDPs. In Al Hudaydah hub, there were new displacements reported in Al Hudaydah city, Al Garrahi, Jabal Ra's and Zabid districts. It was reported that two IDP hosting sites were targeted by air- Total funding strikes in Al Hudaydah city and Hays district which resulted in a number of casualties and injuries required: among sites residents.