COMPARATIVE ECOLOGY of the INVASIVE RUBUS PHOENICOLASIUS and the NATIVE RUBUS ARGUTUS. Anne
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mistaken Identity? Invasive Plants and Their Native Look-Alikes: an Identification Guide for the Mid-Atlantic
Mistaken Identity ? Invasive Plants and their Native Look-alikes an Identification Guide for the Mid-Atlantic Matthew Sarver Amanda Treher Lenny Wilson Robert Naczi Faith B. Kuehn www.nrcs.usda.gov http://dda.delaware.gov www.dsu.edu www.dehort.org www.delawareinvasives.net Published by: Delaware Department Agriculture • November 2008 In collaboration with: Claude E. Phillips Herbarium at Delaware State University • Delaware Center for Horticulture Funded by: U.S. Department of Agriculture Natural Resources Conservation Service Cover Photos: Front: Aralia elata leaf (Inset, l-r: Aralia elata habit; Aralia spinosa infloresence, Aralia elata stem) Back: Aralia spinosa habit TABLE OF CONTENTS About this Guide ............................1 Introduction What Exactly is an Invasive Plant? ..................................................................................................................2 What Impacts do Invasives Have? ..................................................................................................................2 The Mid-Atlantic Invasive Flora......................................................................................................................3 Identification of Invasives ..............................................................................................................................4 You Can Make a Difference..............................................................................................................................5 Plant Profiles Trees Norway Maple vs. Sugar -

Appendix 6: Invasive Plant Species
USDA Forest Service Understanding i-Tree – Appendix 6: Invasive Plant Species APPENDIX 6 Invasive Plant Species The following is a list of invasive tree and shrub species by state that are included in i-Tree database (version 6). Each list of invasive species is followed by the reference of the source which were obtained circa 2014. Some of the Web addresses are no longer working; some have been relocated to alternative sites. State-specific invasive species lists will be updated in the future. Alabama Ailanthus altissima Lonicera japonica Poncirus trifoliate Albizia julibrissin Lonicera maackii Pyrus calleryana Ardisia crenata Lonicera morrowii Rosa bracteata Cinnamomum camphora Lonicera x bella Rosa multiflora Elaeagnus pungens Mahonia bealei Triadica sebifera Elaeagnus umbellata Melia azedarach Vernicia fordii Ligustrum japonicum Nandina domestica Wisteria sinensis Ligustrum lucidum Paulownia tomentosa Ligustrum sinense Polygonum cuspidatum Alabama Invasive Plant Council. 2007. 2007 plant list. Athens, GA: Center for Invasive Species and Ecosystem Health, Southeast Exotic Pest Plant Council. http://www.se-eppc.org/ alabama/2007plantlist.pdf Alaska Alnus glutinosa Lonicera tatarica Sorbus aucuparia Caragana arborescens Polygonum cuspidatum Cytisus scoparius Prunus padus Alaska National Heritage Program. 2014. Non-Native plant data. Anchorage, AK: University of Alaska Anchorage. http://aknhp.uaa.alaska.edu/botany/akepic/non-native-plant-species- list/#content Arizona Alhagi maurorum Rhus lancea Tamarix parviflora Elaeagnus angustifolia Tamarix aphylla Tamarix ramosissima Euryops multifidus Tamarix chinensis Ulmus pumila Arizona Wildland Invasive Plant Working Group. 2005. Invasive non-native plants that threaten wildlands in Arizona. Phoenix, AZ: Southwest Vegetation Management Association https:// www.swvma.org/wp-content/uploads/Invasive-Non-Native-Plants-that-Threaten-Wildlands-in- Arizona.pdf (Accessed Sept 3. -

Non-Native Invasive Plants of the City of Alexandria, Virginia
March 1, 2019 Non-Native Invasive Plants of the City of Alexandria, Virginia Non-native invasive plants have increasingly become a major threat to natural areas, parks, forests, and wetlands by displacing native species and wildlife and significantly degrading habitats. Today, they are considered the greatest threat to natural areas and global biodiversity, second only to habitat loss resulting from development and urbanization (Vitousek et al. 1996, Pimentel et al. 2005). The Virginia Department of Conservation and Recreation has identified 90 non-native invasive plants that threaten natural areas and lands in Virginia (Heffernan et al. 2014) and Swearingen et al. (2010) include 80 plants from a list of nearly 280 non-native invasive plant species documented within the mid- Atlantic region. Largely overlapping with these and other regional lists are 116 species that were documented in the City of Alexandria, Virginia during vegetation surveys and natural resource assessments by the City of Alexandria Dept. of Recreation, Parks, and Cultural Activities (RPCA), Natural Lands Management Section. This list is not regulatory but serves as an educational reference informing those with concerns about non-native invasive plants in the City of Alexandria and vicinity, including taking action to prevent the further spread of these species by not planting them. Exotic species are those that are not native to a particular place or habitat as a result of human intervention. A non-native invasive plant is here defined as one that exhibits some degree of invasiveness, whether dominant and widespread in a particular habitat or landscape or much less common but long-lived and extremely persistent in places where it occurs. -

Invasive Plant List
NON-NATIVE INVASIVE PLANTS OF ARLINGTON COUNTY, VIRGINIA While up to 40% of the plants found in a typical urban environment are non-native species, a relatively small number of these “alien” plants are known to represent an ecological threat to the natural environment (parks, woodlands, and backyards). Known as “invasive species”, these non-natives will spread from urban plantings into natural areas, eliminate native species, alter natural plant communities, and degrade the environment. The following plants have been documented as invasive species in Arlington. Known invasive plant species should not be planted as part of any Arlington County sponsored project. This list will be periodically reviewed by the Invasive Plant Coordinator (DPR) and updated by Version (date). Invasive Plant Species List Acer spp.: campestre, tataricum var. ginnala Hedge, Amur maple Threat Acer spp.: palmatum, plantanoides, pseudoplatanus Japanese, Norway, Sycamore maple Invasive Actinidia arguta Hardy kiwi Threat Aegopodium podagraria Goutweed Invasive Agrostis capillaris Colonial bent-grass Invasive Ailanthus altissima Tree of Heaven Invasive Akebia quinata Five-leaved akebia Invasive Albizia julibrissin Mimosa Invasive Aldrovanda vesiculosa* Waterwheel Threat Alliaria petiolata Garlic mustard Invasive Alternanthera philoxeroides Alligator weed Invasive Ampelopsis brevipedunculata Porcelainberry Invasive Aralia elata Japanese angelica tree Invasive Artemisia vulgaris Mugwort Invasive Arthraxon hispidus var. hispidus Hairy jointgrass Invasive Arum italicum -

Essential Guide to Rubus
The Herb Society of America Essential Guide to Rubus Table of Contents From the Bramble Patch 2 The Brambles: Sorting through the Thicket of Rubus Terminology 3 General Culture 10 Cultivars of Note 12 Rubus as Metaphor: The Bramble Bush and the Law 16 On a Roll with Raspberries (With Recipes) 18 The Traditional Bramble (With Recipes) 21 Blackberry Leaf Tea 24 The Literary Rubus 25 Sources 28 The Herb Society of America, Inc. is dedicated to promoting the knowledge, use, and delight of herbs through educational programs, research, and sharing the experience of its members with the community. Environment Statement The Society is committed to protecting our global environment for the health and well-being of humankind and all growing things. We encourage gardeners to practice environmentally sound horticulture. Medical Disclaimer It is the policy of The Herb Society of America not to advise or recommend herbs for medicinal or health use. This information is intended for educational purposes only and should not be considered as a recommendation or an endorsement of any particular medical or health treatment. Please consult a health care provider before pursuing any herbal treatments. Information is provided as an educational service. Mention of commercial products does not indicate an endorsement by The Herb Society of America. 1 Ghost bramble Photo courtesy of robsplants.com Notes from the Bramble Patch From the blackberry tangled verges along country lanes to the new smaller, thornless raspberries being bred for today’s gardeners, the genus Rubus is a diverse one – feeding us and ornamenting our gardens and providing food and protective cover for wildlife and pollinators alike. -

Rubus Phoenicolasius
www.naturachevale.it [email protected] Nature Integrated Management to 2020 LIFE IP GESTIRE 2020 Rubus phoenicolasius Distribuzione specie (celle 10x10 km) Gestione Facilità gestione/eradicazione Impatti Potenziale gravità impatti Gravità impatti in Lombardia 1. DESCRIZIONE SPECIE a. Taxon (classe, ordine, famiglia): Magnoliopsida, Rosales, Rosaceae. b. Nome scientifico: Rubus phoenicolasius Maxim. c. Nome comune: lampone asiatico. d. Area geografica d’origine: Asia orientale (Cina, Corea e Giappone) e. Habitat d’origine e risorse: R. phoenicolasius è presente nella fascia temperata dell'Asia orientale. Predilige esposizioni soleggiate, su terreni calcarei, ricchi d'humus e con una buona disponibilità idrica. Si trova ai margini dei boschi, nelle radure (es. colonizza nuove aree bene esposte alla luce dopo la caduta di alberi), ai margini di zone umide, in boschi chiari e ai bordi di campi e argini, così come in formazioni prative. In Lombardia si rinviene soprattutto in boschi non gestiti, spesso acidofili (dominanza pino, castagno, faggete). Si può anche trovare ai bordi delle strade, presso vecchie baite (residuo colturale). f. Morfologia e possibili specie simili in Italia o nazioni confinanti: Arbusto alto 1-2 m; fusti arcuati, con sparse spine esili, setole e caratteristici peli ghiandolari rossi, presenti anche nell’infiorescenza e sul picciolo fogliare. Foglie caduche, alterne, composte da 3(-5) foglioline, le laterali subsessili, la terminale (spesso lobata) con peduncolo di 2-3 cm; lamina delle foglioline ovale o rombica, di 4-8×2-5 cm, apice acuto o acuminato, base arrotondata o subcordata, margine irregolarmente seghettato, pagina inferiore grigio-tomentosa, pagina superiore glabra o sparsamente pubescente, di colore verde; stipole lineari, di 5-8 mm. -

Japanese Wineberry Rubus Phoenicolasius
Japanese wineberry Rubus phoenicolasius Description Introduced in the United States in 1890 as breeding stock for new Rubus cultivars (blackberries and raspberries). It is used today by berry breeders to add specific genes to berry varieties or species. Habit Perennial shrub with long arching stems (canes) up to 9 ft in length. Leaves Alternate, palmately compound, 3 heart-shaped serrated leaflets. Stems Arching, known as canes, grows up to 9 ft in length, Upright stems have red gland tipped hairs and small spines. Source: MISIN. 2021. Midwest Invasive Species Information Network. Michigan State University - Applied Spatial Ecology and Technical Services Laboratory. Available online at https://www.misin.msu.edu/facts/detail.php?id=73. Flowers Small, greenish in color with white petals and reddish hairs; blooms in late spring to early summer. Fruits and Seeds Edible raspberry-like fruit, bright red to orange-red in color, multiple drupes and ripens in mid summer. Produces seeds as well. Habitat Native to Japan, Korea, and China. Found in forests, fields, streams and wetland edge habitats, open woods, savannas and prairie habitats. Reproduction By seeds, and through vegetative means including root buds and the sprouting of new plants from where canes touch the soil. Similar Red raspberry (Rubus idaeus), black raspberry (Rubus occidentalis) / (Rubus leucodermis) and blackberry (Rubus fruticosus). Monitoring and Rapid Response Removal of plants by hand-pulling or use a 4-prong spading fork when the soil is moist. Branches and berries should be bagged but the remaining plant material can be left to compost. Sites can be burned or mowed. -
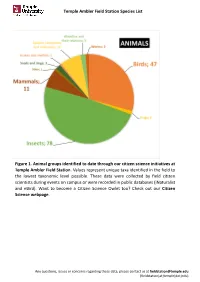
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

28. RUBUS Linnaeus, Sp. P1. 1: 492. 1753. 悬钩子属 Xuan Gou Zi Shu Lu Lingdi (陆玲娣 Lu Ling-Ti); David E
Flora of China 9: 195–285. 2003. 28. RUBUS Linnaeus, Sp. P1. 1: 492. 1753. 悬钩子属 xuan gou zi shu Lu Lingdi (陆玲娣 Lu Ling-ti); David E. Boufford Shrubs or subshrubs, deciduous, rarely evergreen or semievergreen, sometimes perennial creeping dwarf herbs. Stems erect, climbing, arching, or prostrate, glabrous or hairy, usually with prickles or bristles, sometimes with glandular hairs, rarely unarmed. Leaves alternate, petiolate, simple, palmately or pinnately compound, divided or undivided, toothed, glabrous or hairy, sometimes with glandular hairs, bristles, or glands; stipules persistent, ± adnate to petiole basally, undivided or occasionally lobed, persistent or caducous, near base of petiole or at junction of stem and petiole, free, usually dissected, occasionally entire. Flowers bisexual, rarely unisexual and plants dioecious, in cymose panicles, racemes, or corymbs, or several in clusters or solitary. Calyx expanded, some- times with a short, broad tube; sepals persistent, erect or reflexed, (4 or)5(–8). Petals usually 5, rarely more, occasionally absent, white, pink, or red, glabrous or hairy, margin entire, rarely premorse. Stamens numerous, sometimes few, inserted at mouth of hy- panthium; filaments filiform; anthers didymous. Carpels many, rarely few, inserted on convex torus, each carpel becoming a drupelet or drupaceous achene; locule 1; ovules 2, only 1 developing, collateral, pendulous; style filiform, subterminal, glabrous or hairy; stig- ma simple, capitate. Drupelets or drupaceous achenes aggregated on semispherical, conical, or cylindrical torus, forming an aggre- gate fruit, separating from torus and aggregate hollow, or adnate to torus and falling with torus attached at maturity and aggregate solid; seed pendulous, testa membranous; cotyledons plano-convex. -

Natural Resource Condition Assessment, Saratoga National
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Saratoga National Historical Park Natural Resource Condition Assessment Natural Resource Report NPS/NETN/NRR—2014/751 ON THE COVER A Revolutionary War replica cannon overlooking the Hudson River at Saratoga National Historical Park, New York. Photograph by: Rebecca Wagner Saratoga National Historical Park Natural Resource Condition Assessment Natural Resource Report NPS/NETN/NRR—2014/751 Rebecca Wagner, Charles Andrew Cole, Larry Gorenflo, Brian Orland and Ken Tamminga The Pennsylvania State University Department of Landscape Architecture University Park, PA 16802 Margaret C. Brittingham, C. Paola Ferreri and Margot Kaye The Pennsylvania State University Department of Ecosystem Science and Management University Park, PA 16802 January 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -
Rubus Phoenicolasius (Maxim)
Rubus phoenicolasius (Maxim) Rubus phoenicolasius, commonly known as Japanese wineberry is native to temperate Asia; it is widely naturalised in temperate regions of the world. A vigorous shrub, it can form dense thickets covering large areas in invaded habitats displacing native plants. It is found growing in forests, wood, riparian areas, grasslands R. phoenicolasius was introduced into the USA in 1890 as breedingand fields. stock for new raspberry and blackberry cultivars, but escaped from cultivation and is listed as invasive in Maryland, Pennsylvania, Tennessee, Virginia, North Carolina and West Virginia. It is advisable not to plant Japanese wineberry unless it can be contained to prevent unintentional spread. A study (Gorchov et al 2011) that investigated the role of R. phoenicolasius in a deciduous forest in Maryland, USA found thatlocalized R. phoenicolasius disturbance, requiresspecifically disturbances treefalls, suchin the as invasiontreefalls toof establish in forests. However, it was found that established plants can survive under canopy closure leading to stand-wide invasion. The authors observe that managers can prevent invasion by Photo credit: Photo: Wikimedia Commons (User: Qwert1234) monitoring large gaps for new invasions every three years. removed. It is recommended that branches with berries should be Plants can be removed by hand pulling or mechanically if the bagged but the remaining plant material can be left to compost. soil is moist and soft and the roots and any cane fragments are (Spencer, 2009). Reference: Gorchov,L. David, Emily Thompson,Jay O’Neill,Dennis Whigam and Douglas A. Noe, 2011. Treefall gaps required for establishment, but not survival, of invasive Rubus phoenicolasius in deciduous forest, Maryland, USA Spencer, R. -

Wineberry (Rubus Phoenicolasius) Plant Facts Nutritional Benefits
WINEBERRY (RUBUS PHOENICOLASIUS) PLANT FACTS Wineberry (Rubus phoenicolasius) is a mid-size, perennial, berry-producing viney shrub or bramble within the rose family (rosaceae). It is self-fertile and pollinated by insects. It grows and spreads rapidly, with trailing canes usually 4 to 5 feet in length but reaching up to 9 feet in the right conditions. New canes grow liberally from the base of the plant and produce new cloned plants easily at any point where the canes touch soil. The canes are covered in red, glandular (liquid producing) hairs. The sticky liquid produced by the glands resembles that of a carnivorous plant, but seem to serve a defensive purpose in the case of the Wineberry. Both it and the common red raspberry are within the Idaebatus sub-genus of the Rubus genus, perhaps explaining why the Wineberry is also called the wine raspberry. Also known as the Japanese Wineberry, the plant is native to Asia and was cultivated in North America just before the twentieth century. Due to its rapid growth potential and ability to out-compete native plants, it is listed as an invasive species in several States and is commonly found in the Eastern United States from Georgia to Maine and Canada in both rural and urban locations. GROWING INFO Wineberry is a hardy plant that can survive in zones 4-8. It can thrive in a variety of soil conditions, but prefers well-drained and nutrient-rich loam. Although tolerant of part shade (adapted as a forest edge plant), it is most productive in full sun.