Chestermere Lake Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bridging the Gap Also Inside
Suburbs Satellites CALGARY & NEIGHBOURING COMMUNITIES& • MAY 2013 Bridging the gap The charm and benefits of small town living near Calgary. Also inside: Spotlight on Southeast Calgary WWW.CALGARYHERALD.COM/SUBS SUBURBSSUBURBS ++ SATELLITESSATELLITES JUNEMAY 20132012 1 everything WE BUILD, WE BUILD around you. Use the power of Baywest’s red pen to make custom changes to your floorplan that suit you. You know there’s a big distinction between “good enough” and “just We build homes from the $400s’ to $1M+ right” and in that gap, exists the opportunity for something better. You wouldn’t think twice about having a suit tailored to fit you, so why compromise on your most important and personal investment? You should expect more. It’s about floorplanning – and inviting you to be a part of that process. Our clients wouldn’t have it any other way. BUILDING IN THESE FINE COMMUNITIES AUBURN BAY SE • RIVERSTONE of CRANSTON SE • MAHOGANY SE • RANCHES OF SILVERADO SW Connect with us: DRESSAGE OF SILVERADO SW • RANCHERS’ RISE in OKOTOKS • NOLAN HILL NW READY-TO-GO | T AILORED | C U S T OM BAYWESTHOMES.COMBAYWESTHOMES.COM 2 SUBURBS + SATELLITES MAY 2013 WWW.CALGARYHERALD.COM/SUBS in this issue Suburbs 2A 21 Airdrie Family moves to Airdrie for house Satellites DIDSBURY CALGARY & NEIGHBOURING COMMUNITIES& • MAY 2013 prices, stays for the schools. 18 Cochrane 582 Historic Bridging the gap The charm and benefits of small downtown CARSTAIRS town living near Calgary. to play a key 4 Chestermere role in the Planners respond to town’s town’s 2 torrid population growth. Also inside: future plans. -

RURAL ECONOMY Ciecnmiiuationofsiishiaig Activity Uthern All
RURAL ECONOMY ciEcnmiIuationofsIishiaig Activity uthern All W Adamowicz, P. BoxaIl, D. Watson and T PLtcrs I I Project Report 92-01 PROJECT REPORT Departmnt of Rural [conom F It R \ ,r u1tur o A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta W. Adamowicz, P. Boxall, D. Watson and T. Peters Project Report 92-01 The authors are Associate Professor, Department of Rural Economy, University of Alberta, Edmonton; Forest Economist, Forestry Canada, Edmonton; Research Associate, Department of Rural Economy, University of Alberta, Edmonton and Research Associate, Department of Rural Economy, University of Alberta, Edmonton. A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta Interim Project Report INTROI)UCTION Recreational fishing is one of the most important recreational activities in Alberta. The report on Sports Fishing in Alberta, 1985, states that over 340,000 angling licences were purchased in the province and the total population of anglers exceeded 430,000. Approximately 5.4 million angler days were spent in Alberta and over $130 million was spent on fishing related activities. Clearly, sportsfishing is an important recreational activity and the fishery resource is the source of significant social benefits. A National Angler Survey is conducted every five years. However, the results of this survey are broad and aggregate in nature insofar that they do not address issues about specific sites. It is the purpose of this study to examine in detail the characteristics of anglers, and angling site choices, in the Southern region of Alberta. Fish and Wildlife agencies have collected considerable amounts of bio-physical information on fish habitat, water quality, biology and ecology. -

Water Storage Opportunities in the South Saskatchewan River Basin in Alberta
Water Storage Opportunities in the South Saskatchewan River Basin in Alberta Submitted to: Submitted by: SSRB Water Storage Opportunities AMEC Environment & Infrastructure, Steering Committee a Division of AMEC Americas Limited Lethbridge, Alberta Lethbridge, Alberta 2014 amec.com WATER STORAGE OPPORTUNITIES IN THE SOUTH SASKATCHEWAN RIVER BASIN IN ALBERTA Submitted to: SSRB Water Storage Opportunities Steering Committee Lethbridge, Alberta Submitted by: AMEC Environment & Infrastructure Lethbridge, Alberta July 2014 CW2154 SSRB Water Storage Opportunities Steering Committee Water Storage Opportunities in the South Saskatchewan River Basin Lethbridge, Alberta July 2014 Executive Summary Water supply in the South Saskatchewan River Basin (SSRB) in Alberta is naturally subject to highly variable flows. Capture and controlled release of surface water runoff is critical in the management of the available water supply. In addition to supply constraints, expanding population, accelerating economic growth and climate change impacts add additional challenges to managing our limited water supply. The South Saskatchewan River Basin in Alberta Water Supply Study (AMEC, 2009) identified re-management of existing reservoirs and the development of additional water storage sites as potential solutions to reduce the risk of water shortages for junior license holders and the aquatic environment. Modelling done as part of that study indicated that surplus water may be available and storage development may reduce deficits. This study is a follow up on the major conclusions of the South Saskatchewan River Basin in Alberta Water Supply Study (AMEC, 2009). It addresses the provincial Water for Life goal of “reliable, quality water supplies for a sustainable economy” while respecting interprovincial and international apportionment agreements and other legislative requirements. -

Bow River Basin State of the Watershed Summary 2010 Bow River Basin Council Calgary Water Centre Mail Code #333 P.O
30% SW-COC-002397 Bow River Basin State of the Watershed Summary 2010 Bow River Basin Council Calgary Water Centre Mail Code #333 P.O. Box 2100 Station M Calgary, AB Canada T2P 2M5 Street Address: 625 - 25th Ave S.E. Bow River Basin Council Mark Bennett, B.Sc., MPA Executive Director tel: 403.268.4596 fax: 403.254.6931 email: [email protected] Mike Murray, B.Sc. Program Manager tel: 403.268.4597 fax: 403.268.6931 email: [email protected] www.brbc.ab.ca Table of Contents INTRODUCTION 2 Overview 4 Basin History 6 What is a Watershed? 7 Flora and Fauna 10 State of the Watershed OUR SUB-BASINS 12 Upper Bow River 14 Kananaskis River 16 Ghost River 18 Seebe to Bearspaw 20 Jumpingpound Creek 22 Bearspaw to WID 24 Elbow River 26 Nose Creek 28 WID to Highwood 30 Fish Creek 32 Highwood to Carseland 34 Highwood River 36 Sheep River 38 Carseland to Bassano 40 Bassano to Oldman River CONCLUSION 42 Summary 44 Acknowledgements 1 Overview WELCOME! This State of the Watershed: Summary Booklet OVERVIEW OF THE BOW RIVER BASIN LET’S TAKE A CLOSER LOOK... THE WATER TOWERS was created by the Bow River Basin Council as a companion to The mountainous headwaters of the Bow our new Web-based State of the Watershed (WSOW) tool. This Comprising about 25,000 square kilometres, the Bow River basin The Bow River is approximately 645 kilometres in length. It begins at Bow Lake, at an River basin are often described as the booklet and the WSOW tool is intended to help water managers covers more than 4% of Alberta, and about 23% of the South elevation of 1,920 metres above sea level, then drops 1,180 metres before joining with the water towers of the watershed. -
Published Local Histories
ALBERTA HISTORIES Published Local Histories assembled by the Friends of Geographical Names Society as part of a Local History Mapping Project (in 1995) May 1999 ALBERTA LOCAL HISTORIES Alphabetical Listing of Local Histories by Book Title 100 Years Between the Rivers: A History of Glenwood, includes: Acme, Ardlebank, Bancroft, Berkeley, Hartley & Standoff — May Archibald, Helen Bircham, Davis, Delft, Gobert, Greenacres, Kia Ora, Leavitt, and Brenda Ferris, e , published by: Lilydale, Lorne, Selkirk, Simcoe, Sterlingville, Glenwood Historical Society [1984] FGN#587, Acres and Empires: A History of the Municipal District of CPL-F, PAA-T Rocky View No. 44 — Tracey Read , published by: includes: Glenwood, Hartley, Hillspring, Lone Municipal District of Rocky View No. 44 [1989] Rock, Mountain View, Wood, FGN#394, CPL-T, PAA-T 49ers [The], Stories of the Early Settlers — Margaret V. includes: Airdrie, Balzac, Beiseker, Bottrell, Bragg Green , published by: Thomasville Community Club Creek, Chestermere Lake, Cochrane, Conrich, [1967] FGN#225, CPL-F, PAA-T Crossfield, Dalemead, Dalroy, Delacour, Glenbow, includes: Kinella, Kinnaird, Thomasville, Indus, Irricana, Kathyrn, Keoma, Langdon, Madden, 50 Golden Years— Bonnyville, Alta — Bonnyville Mitford, Sampsontown, Shepard, Tribune , published by: Bonnyville Tribune [1957] Across the Smoky — Winnie Moore & Fran Moore, ed. , FGN#102, CPL-F, PAA-T published by: Debolt & District Pioneer Museum includes: Bonnyville, Moose Lake, Onion Lake, Society [1978] FGN#10, CPL-T, PAA-T 60 Years: Hilda’s Heritage, -

Recreational Ecosystem Service Benefits from the Chestermere Lake Reservoir: Value for Day Use Non-Resident Visitors
RECREATIONAL ECOSYSTEM SERVICE BENEFITS FROM THE CHESTERMERE LAKE RESERVOIR: VALUE FOR DAY USE NON-RESIDENT VISITORS ROBERT BEWER B.Sc., University of Saskatchewan, 2003 B.A., University of Saskatchewan, 2004 A Thesis Submitted to the School of Graduate Studies of the University of Lethbridge in Partial Fulfillment of the Requirements for the Degree MASTER OF ARTS Department of Geography University of Lethbridge LETHBRIDGE, ALBERTA, CANADA © Robert Bewer, 2012 Library and Archives Bibliotheque et Canada Archives Canada Published Heritage Direction du 1+1 Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-93720-4 Our file Notre reference ISBN: 978-0-494-93720-4 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distrbute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

TOWN of CHESTERMERE AGENDA for the Regular Meeting of Council to Be Held Tuesday, February 19, 2013 at 6:00Pm in Council Chambers at the Municipal Office
TOWN OF CHESTERMERE AGENDA For the Regular Meeting of Council to be held Tuesday, February 19, 2013 at 6:00pm in Council Chambers at the Municipal Office CALL TO ORDER A. ADOPTION OF AGENDA B. APPOINTMENTS 2 1. Anniversary Park – McElhanney Architects 2. CUI – Rates (Separate Cover) C. ADOPTION OF MINUTES 3-10 1. Regular Council Meeting February 4, 2013 11 D. BUSINESS ARISING OUT OF THE MINUTES E. ACTIONS/DECISIONS 12 1. Cheque Listing - February 12, 2013 13 2. Festivals and Events Tourism and Growth Program 14 3. Anniversary Park 15-16 4. Expropriation of Lift Station 13 – Map of Proposed Location 17 5. Community Recreation Coordinator 18 6. 2013 Local Election Advance Vote 19 7. 2013 Local Election Special Ballots F. BYLAWS 20-23 1. Bylaw 004-13, Amendment to Waterworks Utility Bylaw 004-06 24-27 2. Bylaw 005-13, Amendment to Wastewater Bylaw 007-11 28-30 3. Bylaw 006-13, Amendment to Storm Drainage Bylaw 008-12 31-33 4. Bylaw 007-13, Amendment to Waste Management Bylaw 043-08 34-37 5. Bylaw 003-13, Voter Identification Bylaw G. CORRESPONDENCE & INFORMATION / MINUTES TO BE ACKNOWLEDGED 38-39 1. Council Calendar 40 2. Marigold Library – Municipal Levy for Marigold Library System e 3. AUMA/AMSC February 6, 2013 H. REPORTS 41-42 1. YELL Youth Updates 43-48 2. Protective Services Report I. QUESTION PERIOD J. IN CAMERA K. NEW BUSINESS L. READING FILE M. ADJOURNMENT 2 Regular Council Meeting – February 4, 2013 Page 1 MINUTES OF THE REGULAR COUNCIL MEETING FOR THE TOWN OF CHESTERMERE HELD FEBRUARY 4, 2013 IN COUNCIL CHAMBERS AT THE MUNICIPAL -

Draft Chestermere Lake Recreation Safety Plan
Draft Chestermere Lake Recreation Safety Plan February 26, 2019 Acknowledgements: City of Chestermere Project Team • Kathy Russell, Parks and Recreation Manager • Katelyn Richards, Recreation Coordinator • Hazel Moon, Grant and Research Coordinator • Rosanna Truong, Administrative Assistant to Project Management & Engineering Support • Rami Ajjour, City of Chestermere, Records Clerk • Louise Cox, Watershed Committee, Chair • Cody Cuthill, Watershed Committee, Member • Megan Matthies, City of Chestermere, Communications Strategist • Scott Maurice, Community Peace Officer • Tracey Polowich, City of Chestermere, Finance Consultant • Captain Geoff Standish, Fire Services • Sgt. Garry Tavernier, RCMP External Consulting and Advisory Services • Intelligent Futures- Jeff Robson, Design Lead, John Lewis, President and Founder, Calgary AB • Marsh Risk Consulting - Mark Aeillo, Managing Director, National Practice Leader, Toronto, ON • Michalski Nielsen Associated Limited - Gordon Neilsen, Bracebridge, ON • Transport Canada / Government of Canada - Jason Rosadiuk, Boating Safety Officer, Prairie and North Region (RMEB), Edmonton, AB Table of Contents INTRODUCTION ................................................................................................................................... 5 Lake Recreation Safety Plan ..................................................................................................................................5 What Does a Lake Recreation Safety Plan Do? ....................................................................................................5 -
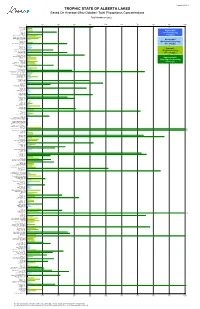
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

'Ice-Free Corridor': Some Recent Results from Alberta
t Pergamon Quaternarylnternational, Vol. 32, pp. 113-126,1996. ~ Copyright © 1996 INQUA/ElsevierScience Ltd. Printed in Great Britain. All rights reserved. 1040-6182/96 $32.00 1040-6182(95)00058-5 LATE QUATERNARY LANDSCAPE HISTORY AND ARCHAEOLOGY IN THE 'ICE-FREE CORRIDOR': SOME RECENT RESULTS FROM ALBERTA Alwynne B. Beaudoin, Milt Wright and Brian Ronaghan Archaeological Survey, Provincial Museum of Alberta, 12845-102nd Avenue, Edmonton, Alberta T5N OM6, Canada Multidisciplinary research programs conducted within the Alberta portion of the 'Ice-Free Corridor' since 1986 as part of the Archaeological Survey's 'First Albertans' project have used studies of palaeogeography and palaeoenvironments to formulate archaeological search strategies. This approach has resulted in the discovery of two new important sites -- Saskatoon Mountain (GhQt-4) and the James Pass Meadow Complex (EkPu-3 to 9) -- that are yielding information on terminal Late Wisconsinan/Early Holocene human occupation of the corridor. Both sites are dated by AMS or conventional radiocarbon dates to the millennium 10,000-9,000 BP. In the fifteen years since the 1978 AMQUA Conference on the Corridor, other researchers have identified four additional significant Paleo-lndian sites in this region. So far, none confirm human occupation of the 'Ice-Free Corridor' region before about 11,000 BP. Copyright © 1996 INQUA/Elsevier Science Ltd INTRODUCTION about Late Wisconsinan glaciation of Alberta -- and particularly the significance and configuration of the 'Ice- In 1986, the Archaeological Survey, Provincial Mu- Free Corridor' -- are changing rapidly (Bobrowsky and seum of Alberta, initiated a study, called the 'First Rutter, 1992; Mandryk, 1992; Rutter et al., 1993). -

PP1 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB MNWH Trout Total L = Bait Allowed Badger Lake OPEN MAY 8 to MAR
Legend: As examples, ‘3 over 63 cm’ indicates a possession and size limit of ‘3 fish each over 63 cm’ or ‘10 fish’ indicates a possession limit of 10 for that species of any size. An empty cell indicates the species is not likely present at that waterbody; however, if caught the default regulations for the Watershed Unit apply. SHL=Special Harvest Licence, BKTR = Brook Trout, BNTR=Brown Trout, BURB = Burbot, CISC = Cisco, CTTR = Cutthroat Trout, DLVR = Dolly Varden, GOLD = Goldeye, LKTR = Lake Trout, LKWH = Lake Whitefish, MNWH = Mountain Whitefish, NRPK = Northern Pike, RNTR = Rainbow Trout, SAUG = Sauger, TGTR = Tiger Trout, WALL = Walleye, YLPR = Yellow Perch. Regulation changes are highlighted blue. Waterbodies closed to angling are highlighted grey. PP1 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB MNWH Trout Total l = Bait allowed Badger Lake OPEN MAY 8 TO MAR. 15 l 0 fish 0 fish 15 fish 10 fish 10 fish Bassano Reservoir See Bow River from Hwy. 24 downstream to Bassano Dam Bullshead Reservoir 28-8-4-W4; Includes Bullhead Creek (outlet) downstream to OPEN APR. 1 TO OCT. 31 Bait ban 1 trout over Eagle Butte Rd. Bridge 50 cm Chestermere Lake OPEN MAY 8 TO MAR. 15 l 3 over 63 cm 15 fish Chin Lakes OPEN MAY 8 TO MAR. 15 l 1 fish 50- 0 fish 10 fish 10 fish 55 cm Clear Lake 14-25-W4 OPEN MAY 8 TO MAR. 15 l 1 over 100 cm Corner Lake OPEN ALL YEAR l 3 fish Cowoki Lake OPEN MAY 8 TO MAR. -

Blue Jay, Vol.31, Issue 3
BREEDING RECORDS “Sl t ^ Of 19 Species Of Birds In Southern Alberta by NORBERT G. KONDLA and HAROLD W. PINEL* >■ * !• Cl )|(t'T Salt and Wilk state “ . there are Frank Lake. The Namaka Lake colony arge gaps in our present knowledge and has been active since at least 1965. arge areas of the province still to be ex- Pied-billed Grebe. Until recently this >lored ornithologically.”4 The purpose bird was unknown as a breeding species >f this article is to provide additional 'in. j in southwestern Alberta. Since 1965, nformation on the breeding status of ’reslj nesting has been documented at Dewin- ertain birds in southern Alberta, ton, Kangienos Lake, Standard, Nor¬ adi specially the area near Calgary. Table 1 man Lake, 20 miles west of Calgary, 8 >rovides details of nesting observations, miles east of Airdrie, Cochrane, and he letters CFNS refer to observation Reesor Lake (Cypress Hills). Birds have orms of the Calgary Field Naturalists also been seen during the breeding Society and the letters PNRS refer to season at Elkwater Lake (Cypress Hills), ards on file with the Prairie Nest : near Chestermere Lake, Priddis, near Records Scheme. We extend our sincere Ghost Lake, Calgary, and Conrich. lanks to observers who have filed data Smith and Wershler reported breeding vith these organizations. Special thanks at Vermilion Lakes and near Turner o to H. W. R. Copland for providing Valley.5 le opportunity to use the files of the rairie Nest Records Scheme and to R. Double-crested Cormorant. A new Fyfe and R. W. Nero for their colony was established on an island at aluable criticisms of the paper.