Functional Response and Predation Potential of Hyperaspis Campestris
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coccinellid Beetles Diversity in Agro-Climatic Zones of Bhubaneswar
Journal of Entomology and Zoology Studies 2017; 5(4): 1244-1248 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Coccinellid beetles diversity in agro-climatic JEZS 2017; 5(4): 1244-1248 © 2017 JEZS zones of Bhubaneswar Received: 09-05-2017 Accepted: 10-06-2017 Sandeep Kumar Mukherjee Sandeep Kumar Mukherjee and Sushree Shailani Suman Associate Professor, Department of Entomology, OUAT, Abstract Bhubaneswar, India The current research was conducted to study the abundance and diversity of various species of Sushree Shailani Suman coccinellid beetles around the agro-climatic zone of Bhubaneswar. It revealed the presence of 10 1) Study Conducted at different species of lady bird beetles viz. E. vigintioctopunctata, B. suturalis, C. transversalis, C. Department of Entomology, undecimpunctata, C. septempunctata, C. sexmaculata, H. maindroni, A. cardoni, S. coccivora and P. OUAT, Bhubaneswar, India dissecta. All total of 1363 numbers of beetles have been collected (few visually counted) from different 2) PhD scholar, KIIT vegetation including vegetables, crop field, fruit orchards, etc. The abundance of P. dissecta species was University, Bhubaneswar, highest (344) contributing about 25.24% of the total population, followed by C. septempunctata (230, Odisha, India 16.87%), and C. transversalis (226, 16.58%). But in terms of species diversity, C. transversalis was the most diversified species among all followed by P. dissecta and E. vigintioctopunctata. The collected species of coccinellid were classified into three groups as per their sub-family viz. Epilachninae, Chilocorinae and Coccinellinae. Among them the coccinellinae sub-family included highest numbers of species (6) with maximum abundance in the area having 701 beetles contributing about 51.42% of all coccinellids collected. -

A Revision of the Coleopterous Family Coccinellid
4T COCCINELLnXE. : (JTambrfljrjr PRINTED BY C. J. CLAY, M.A. AT THE UNIVERSITY PRESS. : d,x A REVISION OF THE COLEOPTEEOUS FAMILY COCCINELLIDJ5., GEORGE ROBERT CROTCH/ M.A. hi Honfcon E. W. JANSON, 28, MUSEUM STREET. 1874. — PREFACE. Having spent many happy hours with the lamented author in the examination of the beautiful forms of which this book treats, I have felt it a pleasant thing to be associated, even in so humble a capacity, with its introduction to the Entomological world ; and the little service I have had the privilege of rendering in the revision of the proof-sheets of the latter half of the work, has been quite a labour of love enabling me to offer a slight testimony of affection to a kind friend, and of my personal interest in that family of the Coleoptera which had first attracted my attention by the singular loveliness of its numerous species. A careful revision by the author himself would have been of incalculable value to the work ; its usefulness would also have been greatly enhanced, had it been possible for him to have made those modifications and additions which his investigations in America afforded materials for. But, of course, this was not possible. There is, however, the conso- lation of knowing that the student can obtain the results of those later researches, in the author's memoir, entitled " Revision of the Coccinellidse of the United States," to which Mr Janson refers in the note which follows this preface. When, in the autumn of 1872, Mr Crotch took his departure for the United States of America, as the first VI PREFACE. -

Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring Within the Kahului Airport Environs, Maui, Hawai‘I: Synthesis Report
Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Prepared by Francis G. Howarth, David J. Preston, and Richard Pyle Honolulu, Hawaii January 2012 Surveying for Terrestrial Arthropods (Insects and Relatives) Occurring within the Kahului Airport Environs, Maui, Hawai‘i: Synthesis Report Francis G. Howarth, David J. Preston, and Richard Pyle Hawaii Biological Survey Bishop Museum Honolulu, Hawai‘i 96817 USA Prepared for EKNA Services Inc. 615 Pi‘ikoi Street, Suite 300 Honolulu, Hawai‘i 96814 and State of Hawaii, Department of Transportation, Airports Division Bishop Museum Technical Report 58 Honolulu, Hawaii January 2012 Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i Copyright 2012 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 1085-455X Contribution No. 2012 001 to the Hawaii Biological Survey COVER Adult male Hawaiian long-horned wood-borer, Plagithmysus kahului, on its host plant Chenopodium oahuense. This species is endemic to lowland Maui and was discovered during the arthropod surveys. Photograph by Forest and Kim Starr, Makawao, Maui. Used with permission. Hawaii Biological Report on Monitoring Arthropods within Kahului Airport Environs, Synthesis TABLE OF CONTENTS Table of Contents …………….......................................................……………...........……………..…..….i. Executive Summary …….....................................................…………………...........……………..…..….1 Introduction ..................................................................………………………...........……………..…..….4 -
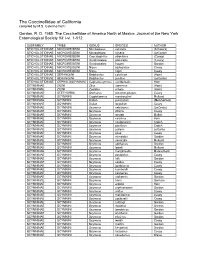
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Coleoptera) John R
NProceedingsotes oN Names of the of hHawaiianawaiiaN e CntomologicalocciNellidae society (2014) 46:1–7 1 Nomenclatural and Taxonomic Notes on Names of Hawaiian Coccinellidae (Coleoptera) John R. Leeper University of Hawaii Insect Museum, 310 Gilmore Hall, 3050 Maile Way, Honolulu, Hawaii 96822; [email protected] Abstract. Hawaii has a long and successful history of coccinellid introductions for biological control of pest insects and powdery mildew. This paper discusses the names of five established species Chilocorus( nigrita (Fabricius), Hippodamia quinquesignata ambigua LeConte, Psyllobora vigintimaculata (Say), Sasajis- cymnus anomalus (Chapin), and Scymnus ambulans Blackburn), giving spelling corrections, changed generic combinations, and one new combination (Sasajis- cymnus anomalus (Chapin), n. comb.) to allow accurate usage of these names in the Hawaiian literature. Justification is also provided for the use of Hyperaspis pantherina Fursch which, until Nishida (2002), appeared in Hawaiian literature as Hyperaspis jocosa (Mulsant). Key words: Coccinellidae, name changes, Sasajiscymnus anomalus (Chapin) N. comb. In the process of updating an earlier (1989) and Booth (1998). The third name review of Hawaiian Coccinellidae (Leeper change not currently recognized by ITIS, 1976) a number of name changes were Scymnus ambulans Blackburn, is based found that have not appeared in Hawai- on a recent determination (Slipinski et al. ian literature, primarily the Proceedings 2012). Effort was also made to reference of the Hawaiian Entomological Society the authorities making the name changes (https://scholarspace.manoa.hawaii.edu/ followed by the names historically used handle/10125/25), Nishida (2002), and within Hawaiian literature. the University of Hawaii Insect Museum (UHIM) Insect Holdings Database (http:// Chilocorus nigrita (Fabricius 1798). -

History of the Biodiversity of Ladybirds (Coccinellidae) at the Black Sea Coast of the Russian Caucasus in the Last 120 Years—
insects Article History of the Biodiversity of Ladybirds (Coccinellidae) at the Black Sea Coast of the Russian Caucasus in the Last 120 Years—Does the Landscape Transformation and Establishment of Harmonia axyridis Have an Impact? Andrzej O. Bie ´nkowskiand Marina J. Orlova-Bienkowskaja * A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 21 November 2020; Published: 23 November 2020 Simple Summary: Studies of the history of regional insect fauna are important for understanding the changes in ecosystems and are therefore crucial for conservation decisions. The harlequin ladybird is a global invader that causes the decline of native ladybirds in some countries. Therefore, it is advisable to monitor the ladybird fauna in regions recently occupied by this species. We analyzed the dynamics of the fauna at the main sea resort of Russia over a period of 120 years to determine the following: (1) how the ladybird biodiversity changed during the intensive landscape transformation; (2) what alien species introduced for pest control have occurred to date; and (3) what the impact is of the harlequin ladybird on the ladybird fauna. We examined specimens collected by us and 54 other collectors including specimens from old museum collections and reconstructed the history of the biodiversity like a picture from puzzle pieces. Surprisingly, landscape transformation did not cause a decrease but rather an increase in ladybird biodiversity; most of the species recorded before 1930 have occurred to date, and 23 other species have spread to the region. -

1902 Governor's Report of the Commissioner of Agriculture And
Report of the Board of Commissioners of Agriculture and Forestry of the Territory of Hawaii for the period ... Hawaii. Honolulu. http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain http://www.hathitrust.org/access_use#pd We have determined this work to be in the public domain, meaning that it is not subject to copyright. Users are free to copy, use, and redistribute the work in part or in whole. It is possible that current copyright holders, heirs or the estate of the authors of individual portions of the work, such as illustrations or photographs, assert copyrights over these portions. Depending on the nature of subsequent use that is made, additional rights may need to be obtained independently of anything we can address. Generated on 2015-11-13 00:46 GMT / http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain / http://www.hathitrust.org/access_use#pd B433330 Generated on 2015-11-13 00:46 GMT / http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain / http://www.hathitrust.org/access_use#pd t r ( / J 7' ZItR Generated on 2015-11-13 00:46 GMT / http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain / http://www.hathitrust.org/access_use#pd OF THEI FOR THE A! Zr f~ TT PPRI NT 1903. I I I 1: a 'r Evs&Nl- Uiw; hai-ivl' 4jim I Generated on 2015-11-13 00:46 GMT / http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain / http://www.hathitrust.org/access_use#pd vi, /9 '-V 4i:", — lk Generated on 2015-11-13 00:46 GMT / http://hdl.handle.net/2027/miun.acj0341.1902.001 Public Domain / http://www.hathitrust.org/access_use#pd REPORT OF THE Commissioner of Agriculture and Forestru, HTONOTLULT, TERRITORY OF HAAIIH, June 30, 1902. -

Download Download
January 31 2020 INSECTA 4 urn:lsid:zoobank. A Journal of World Insect Systematics org:pub:610643CE-3740-4750- UNDI M 8553-2C7B0F0FCE94 0745 New state records of lady beetles (Coleoptera: Coccinellidae: Coccinellinae) from Missouri and Mississippi, USA Louis S. Hesler USDA Agricultural Research Service North Central Agricultural Research Laboratory 2923 Medary Avenue Brookings, SD 57006-9401, USA Date of issue: January 31, 2020 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Louis S. Hesler New state records of lady beetles (Coleoptera: Coccinellidae: Coccinellinae) from Missouri and Mississippi, USA Insecta Mundi 0745: 1–4 ZooBank Registered: urn:lsid:zoobank.org:pub:610643CE-3740-4750-8553-2C7B0F0FCE94 Published in 2020 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book reviews or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources, including the Zoological Record and CAB Abstracts. Insecta Mundi is published irregularly throughout the year, with completed manuscripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. -

Additions to the <I>Hyperaspis</I> Chevrolat
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 3-18-2011 Additions to the Hyperaspis Chevrolat (Coleoptera: Coccinellidae) fauna of South American, descriptions of nine new species, and recognition of Hyperaspis pectoralis Crotch as a valid species Robert D. Gordon Northern Plains Entomology, Willow City, ND 58384, [email protected] F. Guillermo González Santiago, CHILE, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Gordon, Robert D. and González, F. Guillermo, "Additions to the Hyperaspis Chevrolat (Coleoptera: Coccinellidae) fauna of South American, descriptions of nine new species, and recognition of Hyperaspis pectoralis Crotch as a valid species" (2011). Insecta Mundi. 679. https://digitalcommons.unl.edu/insectamundi/679 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0160 Additions to the Hyperaspis Chevrolat (Coleoptera: Coccinellidae) fauna of South American, descriptions of nine new species, and recog- nition of Hyperaspis pectoralis Crotch as a valid species Robert D. Gordon Northern Plains Entomology P. O. Box 65 Willow City, ND 58384, USA F. Guillermo González Nocedal 6455 La Reina Santiago, CHILE Date of Issue: March 18, 2011 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Robert D. Gordon and F. Guillermo González Additions to the Hyperaspis Chevrolat (Coleoptera: Coccinellidae) fauna of South American, descriptions of nine new species, and recognition of Hyperaspis pectoralis Crotch as a valid species Insecta Mundi 0160: 1-20 Published in 2011 by Center for Systematic Entomology, Inc. -

Dactylopius Opuntiae (Hemiptera: Dactylopiidae) on 2 Moroccan Resistant Cactus Germplasm
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.23.453565; this version posted July 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Life history of Dactylopius opuntiae (Hemiptera: Dactylopiidae) on 2 Moroccan resistant cactus germplasm 3 4 SHORT TITLE: Characterization of resistance of cactus genotypes to 5 Dactylopius opuntiae 6 7 Mohamed El Aalaoui,1* and Mohamed Sbaghi1 8 9 1National Institute of Agricultural Research, Ennasr Rabat, Morocco BP 415 RP Rabat, Morocco 10 11 *Corresponding author. Email: [email protected]. 12 13 These authors contributed equally to this work. 14 15 16 17 18 19 20 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.23.453565; this version posted July 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 22 Abstract 23 The important damages caused by Dactylopius opuntiae (Hemiptera: Dactylopiidae) to cactus crops 24 around the world require an integrated pest management (IPM) approach, based on the combination 25 of several techniques (varietal resistance, biological, chemical methods, etc). In this sense, this study 26 evaluated the resistance of 10 Moroccan cactus genotypes to D. opuntiae in order to characterize the 27 expression of antixenosis and/or antibiosis. -

An Annotated List of Insects and Other Arthropods
This file was created by scanning the printed publication. Text errors identified by the software have been corrected; however, some errors may remain. Invertebrates of the H.J. Andrews Experimental Forest, Western Cascade Range, Oregon. V: An Annotated List of Insects and Other Arthropods Gary L Parsons Gerasimos Cassis Andrew R. Moldenke John D. Lattin Norman H. Anderson Jeffrey C. Miller Paul Hammond Timothy D. Schowalter U.S. Department of Agriculture Forest Service Pacific Northwest Research Station Portland, Oregon November 1991 Parson, Gary L.; Cassis, Gerasimos; Moldenke, Andrew R.; Lattin, John D.; Anderson, Norman H.; Miller, Jeffrey C; Hammond, Paul; Schowalter, Timothy D. 1991. Invertebrates of the H.J. Andrews Experimental Forest, western Cascade Range, Oregon. V: An annotated list of insects and other arthropods. Gen. Tech. Rep. PNW-GTR-290. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 168 p. An annotated list of species of insects and other arthropods that have been col- lected and studies on the H.J. Andrews Experimental forest, western Cascade Range, Oregon. The list includes 459 families, 2,096 genera, and 3,402 species. All species have been authoritatively identified by more than 100 specialists. In- formation is included on habitat type, functional group, plant or animal host, relative abundances, collection information, and literature references where available. There is a brief discussion of the Andrews Forest as habitat for arthropods with photo- graphs of representative habitats within the Forest. Illustrations of selected ar- thropods are included as is a bibliography. Keywords: Invertebrates, insects, H.J. Andrews Experimental forest, arthropods, annotated list, forest ecosystem, old-growth forests. -

The Biodiversity of Terrestrial Arthropods in Madeira and Selvagens Manual Versión Española
Revista IDE@ - SEA, nº 6B (30-06-2015): 1–20. ISSN 2386-7183 1 Ibero Diversidad Entomológica @ccesible www.sea-entomologia.org/IDE@ Introduction The biodiversity of terrestrial arthropods in Madeira and Selvagens Manual Versión española The biodiversity of terrestrial arthropods in Madeira and Selvagens archipelagos Mário Boieiro1,2, António Franquinho Aguiar3, Carla Rego1,2, Paulo A.V. Borges1,2 & Artur R.M. Serrano4 1 cE3c – Centre for Ecology, Evolution and Environmental Changes / Azorean Biodiversity Group and Universidade dos Açores - Departamento de Ciências Agrárias, 9700-042 Angra do Heroísmo, Açores, Portugal. 2 CITA-A and Portuguese Platform for Enhancing Ecological Research & Sustainability (PEERS). 3 Núcleo de Entomologia, Laboratório Agrícola da Madeira, 9135-372 Camacha, Madeira, Portugal. 4 cE3c – Centre for Ecology, Evolution and Environmental Changes / Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal. 1. The archipelagos of Madeira and Selvagens The oceanic archipelagos of Madeira and Selvagens are located in the eastern Atlantic, between 30-33ºN and 15-17ºW, being part of the Macaronesia biogeographical region. The archipelago of Madeira is composed of Madeira Island, Porto Santo Island and its surrounding islets, and the Desertas islands, which include Deserta Grande, Bugio and Ilhéu Chão (Fig. 1). This archi- pelago is distanced from the Iberian Peninsula by 1000 km, but its distance to the nearest mainland (coast of Morocco) is just 600 km. All the islands of Madeira archipelago are volcanic in origin and have originat- ed from a single volcanic building – the Madeira-Porto Santo complex. The rugged orography and the altitudinal span of Madeira Island led to the occurrence of some natu- ral habitat-types which are distributed along the altitudinal gradient (see Plate I).