Sol-Gel Reaction Is the Best Way from Ancient Inorganic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ART and ARCHAEOLOGY Vocabulary ART and ARCHAEOLOGY Vocabulary Version 1.1 (Last Updated: 2018-01-22)
- Institute for Scientific and Technical Information - ART and ARCHAEOLOGY Vocabulary ART and ARCHAEOLOGY Vocabulary Version 1.1 (Last updated: 2018-01-22) Controlled vocabulary used for indexing bibliographical records for the "Art and Archaeology" FRANCIS database (1972-2015, http://pascal-francis.inist.fr/ ). This resource contains 1960 entries grouped into 133 collections. A French version of this resource is also available. The resource is browsable online on the terminological portal Loterre: https://www.loterre.fr Legend • Syn: Synonym. • → : Corresponding Preferred Term. • FR: French Preferred Term. • ES: Spanish Preferred Term. • DE: German Preferred Term. • BT: Broader Term. • SC: Semantic Category. • DO: Subject Field. • URI: Concept's URI (link to the online view). This resource is licensed under a Creative Commons Attribution 4.0 International license: TABLE OF CONTENTS Alphabetical Index 4 Terminological Entries 5 List Of Entries 140 Collections 183 Alphabetical Index from 'Abd al-Samad to 'Abd al-Samad p. 6 -6 from 10th century AD to 1st millenium BC p. 9 -9 from 20th century AD to 2nd millenium BC p. 11 -11 from 30th century BC to 3rd millenium BC p. 12 -12 from 4th century AD to 4th millenium BC p. 13 -13 from 5th century AD to 5th millenium BC p. 14 -14 from 6th century AD to 6th dynasty p. 15 -15 from 7th century AD to 7th dynasty p. 16 -16 from 8th century AD to 8th dynasty p. 17 -17 from 9th century AD to 9th dynasty p. 18 -18 from Abassid dynasty to Aztec Civilization p. 19 -27 from baboon to Byzantium p. -

Tracking the Neolithic House in Europe Sedentism, Architecture, and Practice One World Archaeology
One World Archaeology Daniela Hofmann Jessica Smyth Editors Tracking the Neolithic House in Europe Sedentism, Architecture, and Practice One World Archaeology Series Editors: Heather Burke, Flinders University of South Australia, Australia Gustavo Politis, Universidad Nacionaldel Centro, Buenos Aires, Argentina Gabriel Cooney, University College, Dublin, Ireland For further volumes: http://www.springer.com/series/8606 Daniela Hofmann · Jessica Smyth Editors Tracking the Neolithic House in Europe Sedentism, Architecture and Practice 1 3 Editors Daniela Hofmann Jessica Smyth School of History, Archaeology and Religion School of Chemistry Cardiff University University of Bristol Cardiff Bristol United Kingdom United Kingdom ISBN 978-1-4614-5288-1 ISBN 978-1-4614-5289-8 (eBook) DOI 10.1007/978-1-4614-5289-8 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2012954540 © Springer Science+Business Media New York 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recita- tion, broadcasting, reproduction on microfilms or in any other physical way, and transmission or infor- mation storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar meth- odology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplica- tion of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. -

ART and ARCHAEOLOGY Vocabulary ART and ARCHAEOLOGY Vocabulary Version 1.1 (Last Updated : Jan
- Institute for scientific and technical information - ART and ARCHAEOLOGY Vocabulary ART and ARCHAEOLOGY Vocabulary Version 1.1 (Last updated : Jan. 22, 2018) This resource contains 1960 entries. Controlled vocabulary used for indexing bibliographical records for the "Art and Archaeology" FRANCIS database (1972-2015, http://pascal-francis.inist.fr/ ). This vocabulary is browsable online at: https://www.loterre.fr Legend • Syn: Synonym. • →: Corresponding Preferred Term. • FR: French Preferred Term. • ES: Spanish Preferred Term. • DE: German Preferred Term. • URI: Concept's URI (link to the online view). This resource is licensed under a Creative Commons Attribution 4.0 International license: LIST OF ENTRIES List of entries English French Page • 10th century AD Xe siècle apr. J.-C. 46 • 10th dynasty Xe dynastie 46 • 11th century AD XIe siècle apr. J.-C. 46 • 11th century BC XIe siècle av. J.-C. 46 • 11th dynasty XIe dynastie 46 • 12th century AD XIIe siècle apr. J.-C 46 • 12th century BC XIIe siècle av. J.-C 46 • 12th dynasty XIIe dynastie 46 • 13th century AD XIIIe siècle apr. J.-C 46 • 13th century BC XIIIe siècle av. J.-C 46 • 13th dynasty XIIIe dynastie 46 • 14th century AD XIVe siècle apr. J.-C 46 • 14th century BC XIVe siècle av. J.-C 46 • 14th dynasty XIVe dynastie 46 • 15th century AD XVe siècle apr. J.-C 46 • 15th century BC XVe siècle av. J.-C 46 • 15th dynasty XVe dynastie 46 • 16th century AD XVIe siècle apr. J.-C 46 • 16th century BC XVIe siècle av. J.-C 46 • 16th dynasty XVIe dynastie 46 • 17th century AD XVIIe siècle apr. -

ARCH202 History of Architecture Spring 2013-2014
University of Nizwa College of Engineering & Architecture Dept. of Architecture & Interior Design ARCH202 History of Architecture Spring 2013-2014 Dr. Janon Kadhim Associate Professor of Architecture ARCH 202 History of Architecture Lecture #2 Mesopotamian ARCHITECTURE Dr. Janon A. W. Kadhim Associate Professor of Architecture University of Nizwa Spring 2013-2014 Mesopotamia: The Cradle of civilization • The caves in Northern • On the land of Mesopotamia contain Mesopotamia we can remains of the ancient trace the growth and Cavemen. development of human • The first Agrarian existence and settlements in the civilization from the world formed in the earliest times in a north of Mesopotamia continuous sequence. some of which go back to 10000 BC. Dr. Janon Kadhim 3 The fertile Crescent Arab Gulf Mesopotamia, the land between the rivers, is situated in the region called the fertile crescent Dr. Janon Kadhim 4 Mesopotamia: Attributes of the Natural Environment • Mesopotamia is part of what is known as the “Fertile Crescent”; • It contains some of the most fertile and rich land in the world; • Diverse topography and climate: which provided a variety of building materials, and led to the creation of a variety of art forms and architectural types; • Mesopotamia does not have any natural means of fortification other than the mountains that create the North and North-Eastern boundaries of the region. This has created a continuous problem for Mesopotamia over history causing it to be invaded many times over history by surrounding entities. Dr. Janon Kadhim 5 Other Natural attributes: • Characterized by the two large rivers, The Tigris and the Euphrates, and their many tributaries; • The rivers are known for their annual floods. -

The Sumerian Culture
Subject : Ancient Scripts and Languages Article: 48 The Sumerian Culture Doç. Dr. Haluk Berkmen I have already given strong clues about the relationship between Turkish and Sumerian. See article 42-The Sumerian Language (1). Sumerians settled in the south of Mesopotamia some 5,500 years ago, but they spread far up to Anatolia. All regional languages are offspring of Sumerian. Sumerian language is one of the earliest known written languages. It is claimed that Sumerian is an isolate language, but the language is agglutinative and originates from Central Asia. It preserves the word root intact while expressing various grammatical changes by adding prefixes, infixes and suffixes. The difference in the noun gender does not exist as it does in the Indo-European and Semitic languages. These characteristics are valid for all Ural-Altaic languages. The Sumerians wrote on soft clay tablets with a stick or wedge-shaped stylus. The "proto-literate" period of Sumerian writing spans c. 3300 to 3000 BC. Records with unambiguously linguistic content, identifiably Sumerian, are those found at Jemdet Nasr, dating to the 31st or 30th century BC. This cuneiform ("wedge-shaped") mode of writing co- existed with the pre-cuneiform ideographic mode. Ideograms have an abstract or conventional meaning, no longer displaying a clear pictorial link with external reality. Above we see how the Sumerian cuneiform script transformed from recognizable pictographic ideographic forms. It is interesting to observe the transformation that occurred between 3,200 BC - 1,000 BC. There are many words that are identical between Turkish and Sumerian, both in their meaning and also in their pronunciation. -

Packaging Through Time
Rewiev article Stanislav Bolanča, Nikola Mrvac, Martina Hajdek PACKAGING THROUGH TIME PACKAGING THROUGH TIME Stanislav Bolanča, Nikola Mrvac, Martina Hajdek University of Zagreb, Faculty of Graphic Arts The task of packaging Materials and packaging in the past Packaging can be briefly defined as a Packaging is a formed material that goods are science, method and technology for product wrapped in or placed within.[1] Packaging preservation. The function of packaging is was in every historical period adapted to to protect the product that is packed in it the civilizational and technological level and to protect the environment from the of the society.[11] There is information on content inside the packaging. As a result, the packaging from 12 000 years ago. It was used products can be gaseous, liquid or solid.[1–3] by people who lived as nomads, hunters, and The packaging must be suitable for use, i.e. gatherers of fruits.[4] At that time, hollow handling, transport and distribution. It should trees, tickles, animal bladders and skin were inform the potential and the actual buyer and used as packaging materials. Flexible leaves it should “sell the product”. [4,5] were joined with flexible grass. Later, leather An important part of the packaging design is reusable bags and wicker bags were made. the material from which it will be made. The [1] Today, people who are at a low level of basic conditions that packaging must meet are development use leaves, leather and wicker the following: strength on material pressure, products as packaging. elasticity, plasticity, stiffness and resistance to After the mentioned materials, materials with strain, toughness, mechanical endurance and new and better properties were found. -
Mortuary Practice, Population Dynamics, and Chronology at the Carrowkeel Passage Tomb Complex, Co
Rites of Passage: Mortuary Practice, Population Dynamics, and Chronology at the Carrowkeel Passage Tomb Complex, Co. Sligo, Ireland By THOMAS KADOR1, LARA M. CASSIDY2, JONNY GEBER3, ROBERT HENSEY4, PÁDRAIG MEEHAN5, and SAM MOORE6 [ABS]The first detailed investigation of the human remains from the Carrowkeel passage tomb complex since their excavation in 1911 has revealed several new and important insights about life, death, and mortuary practice in Neolithic Ireland. Osteological analysis provides the first conclusive proof for the occurrence of dismemberment of the dead at Irish passage tombs, practised contemporarily with cremation as one of a suite of funerary treatments. The research also highlights changes in burial tradition at the complex over the course of the Neolithic. Providing a chronology for these changes allows them to be linked to wider trends in monument construction, which may relate to changes in both land use and climate during the period. Multi-isotope analysis hints at the presence of non-local individuals among the interred and the possible existence of different food sourcing areas at the onset of the later Neolithic period. Preliminary results from ancient DNA sequencing of six individuals from Carrowkeel provide evidence for the genetic ancestry of Irish Neolithic populations, demonstrating their Anatolian origins and links along the Atlantic façade. Keywords: Neolithic, body processing, dismemberment, isotope analysis, aDNA, chronological modelling, passage grave, Ireland On 14 April 1911, a team assembled by R.A.S. Macalister (1870–1950), Professor of Celtic Archaeology at University College Dublin, commenced archaeological investigations at Carrowkeel, Co. Sligo, Ireland. Over 18 days, between April and October, they investigated 14 stone cairns (referred to as ‘carns’). -

Sumer Sumer Was an Ancient Chalcolithic Civilization That Saw Its Artistic Styles Change Throughout Different Periods in Its
Sumer Sumer was an ancient Chalcolithic civilization that saw its artistic styles change throughout different periods in its history. Figure 1: Stele of Vultures Battle formations on a fragment of the Stele of the Vultures. Example of Sumerian pictorial cuneiform writing. The surplus of storable food created by the Eridu economy required an extensive labour force and division of labor with many specialized arts and crafts. In the early Sumerian period, pictograms suggest the abundance of pottery and other artistic traditions. By the late 4th millennium BCE, Sumer was divided into about a dozen independent city-states delineated by canals and other boundary makers. The Sumerian city states rose to power during the prehistorical Ubaid and Uruk periods. Note: Cuneiform is one of the earliest known forms of written expression. Emerging in Sumer around the 30th century BC, with predecessors reaching into the late 4th millennium (the Uruk IV period), cuneiform writing began as a system of pictographs. The Chalcolithic period, or Copper Age, is a phase of the Bronze Age in which metallurgists did not yet know about how to add tin to copper to form bronze during smelting. The Copper Age was originally defined as a transition between the Neolithic and the Bronze Age. Theocratic refers to a theocracy, which is a form of government in which the ruler is recognized as a deity or a deity’s representative. Government officials are regarded as divinely guided, and policy may be pursuant to the doctrine of a particular religion or religious group. Source URL: https://www.boundless.com/art-history/art-ancient-near-east/mesopotamia/sumer/ Saylor URL: http://www.saylor.org/courses/ARTH110#2.1 Attributed to: Boundless www.saylor.org Page 1 of 4 Figure 2: Sumer Map of the cities of Sumer. -
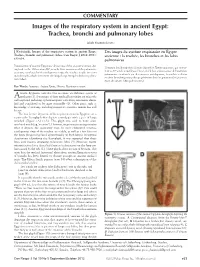
Images of the Respiratory System in Ancient Egypt: Trachea, Bronchi and Pulmonary Lobes
COMMENTARY Images of the respiratory system in ancient Egypt: Trachea, bronchi and pulmonary lobes Jakub Kwiecinski MSc J Kwiecinski. Images of the respiratory system in ancient Egypt: Des images du système respiratoire en Égypte Trachea, bronchi and pulmonary lobes. Can Respir J 2012;19(5): ancienne : la trachée, les bronches et les lobes e33-e34. pulmonaires Examination of ancient Egyptians’ depictions of the respiratory tract, dat- L’examen des descriptions du tube digestif en Égypte ancienne, qui remon- ing back to the 30th century BC, reveals their awareness of the pulmonary tent au 30e siècle avant Jésus-Christ, révèle leur connaissance de l’anatomie anatomy: reinforced with cartilaginous rings, the trachea is split into two pulmonaire : renforcée par des anneaux cartilagineux, la trachée se divise main bronchi, which then enter the lungs (lungs being divided into pulmo- en deux bronches principales qui pénètrent dans les poumons (les poumons nary lobes). étant divisés en lobes pulmonaires). Key Words: Anatomy; Ancient Egypt; History; Respiratory system ncient Egyptians were the first to create an elaborate system of Amedicine (1). Some parts of their medical knowledge are relatively well explored, including a pharmacopoeia with many treatments identi- fied and considered to be quite reasonable (1). Other parts, such as knowledge of anatomy, including respiratory anatomy, remain less well known. The best known depiction of the respiratory tract in Egyptian art is a particular hieroglyph that depicts a windpipe with a pair of lungs attached (Figure 1A) (1-3). This glyph was used to write some unrelated words (eg, ‘to unite’). However, its primary meaning remains what it depicts: the respiratory tract. -

17:00 British Museum, London Britain at 3000 BC
Neolithic Studies Group Autumn Meeting 2011 Monday 7 November 2011 10:30 – 17:00 British Museum, London Britain at 3000 BC Organizers: Timothy Darvill, Josh Pollard, and Kenny Brophy In the centuries either side of 3000 BC the Neolithic communities across the British Isles seem to have undergone fundamental changes marking what some have previously called the Middle Neolithic. The last quarter of the 4th millennium BC sees the appearance of new styles of ceramic - Peterborough and Impressed pottery in southern Britain and Grooved ware in the far north – along with other distinctive forms of material culture such as transverse arrowheads, edge-polished implements and antler maceheads. The first henges appear. There is an explosion of passage graves in size and distribution. Single graves and then cremation cemeteries become widespread. Traditional monuments such as long barrows, oval barrows, causewayed enclosures, and cursuses cease to be built, but many remain the focus of rather different ceremonies and practices. Houses and settlements change form, and there is a whiff of greater regionalization and shifting patterns of contact with the near continent. Environmental changes have been suggested, and there are shifts in settlement practices in some areas – perhaps more dispersed, with less evidence of aggregation. Natural catastrophies may have contributed to social change. The period 3300– 2800 BC is one that has received little attention in recent years and the aim of this meeting is to review the evidence in the light of recent work and new discoveries. Does a middle Neolithic exist and, if so, how might it be defined? Can we trace the origin of new traditions? Was this a period of major religious and ideological change, or simply a time that witnessed reworking of existing values of earlier Neolithic ancestry? Practical detail: New security arrangements at the British Museum mean that it is no longer possible to take cases measuring more than 40x40x50cm/8kg, or any wheeled cases, into the museum. -

The First Shoulder Replacement in Ancient Greek Mythology: the Story of Pelops, King of Elis
Orthopaedics & Traumatology: Surgery & Research 105 (2019) 801–803 Contents lists available at ScienceDirect Orthopaedics & Traumatology: Surgery & Research jou rnal homepage: www.elsevier.com Pioneers in surgery The first shoulder replacement in Ancient Greek Mythology: The story of Pelops, King of Elis a,∗ a,b Nikitas N. Nomikos , Chris K. Yiannakopoulos a Faculty of Physical Education and Sports Science, National and Kapodistrian University of Athens (NKUA), Athens, Greece b Iaso General Hospital, 264, Mesogeion Avenue, 15562 Holargos, Greece a r t i c l e i n f o a b s t r a c t Article history: Medical science and the need for the treatment of diseases and injuries, is as old as human history. This Received 11 December 2018 research aims to identify and study the first registered shoulder replacement in Ancient Greek Mythology. Accepted 3 April 2019 After a review of literature, the texts that referred to the myth of Pelops’ ivory shoulder were identified and analysed, as were the texts which contain the first citation for shoulder replacement in the prehistoric Keywords: era. Pelops © 2019 Elsevier Masson SAS. All rights reserved. Trauma Shoulder Prosthetics Replacement Arthroplasty 1. Introduction of intracranial pressure and decompression of the head by cran- iotomy. An example of the above mentioned myth (which contains The beginning of medical science is lost in the depths of human a scenario of divine intervention) can be seen in the illustration history. Indications for medical cases exist from ancient times. of an Archaic Greek, lip-cup [8], which shows the birth of Athena Nascent prosthetic science was beginning to emerge in the Nile val- following the blow to head of Zeus from Hephaestus [7]. -

Observing Small Fiber Dysfunction Using Nociceptive Stimulus Detection and Evoked Potentials
Master’s thesis Technical Medicine University of Twente Observing small fiber dysfunction using nociceptive stimulus detection and evoked potentials Explorative investigations with the novel NDT-EP measurement method in a lidocaine model and diabetes mellitus patients S.R. (Silvano) Gefferie, BSc. May 25th, 2020 Observing small fiber dysfunction using nociceptive stimulus detection and evoked potentials Explorative investigations with the novel NDT-EP measurement method in a lidocaine model and diabetes mellitus patients By Silvano R. Gefferie May 25th, 2020 A thesis submitted to the University of Twente in partial fulfillment of the requirements for the degree of Master of Science in Technical Medicine Examination committee Chairman - P.H. (Peter) Veltink, PhD University of Twente Technical supervisor - J.R. (Jan) Buitenweg, PhD University of Twente Medical supervisor - I.P. (Imre) Krabbenbos, MD St. Antonius Hospital Process supervisor - R.J. (Rian) Haarman, MSc. University of Twente External member - C.J.M. (Carine) Doggen, PhD University of Twente Technical Medicine (Medical Sensing and Stimulation) Faculty of Science and Technology University of Twente Enschede, The Netherlands (Lieberman, 2018)(Yagihashi et al., 2007) Preface After one year of hard work at the St. Antonius Hospital, I can finally present this thesis. This report aims to inform the reader about the study that I conducted during my graduation internship, being the concluding piece of seven years in the Technical Medicine program (University of Twente). As of June 2019, I started this graduation internship at the Department of Anesthesiology, Intensive Care and Pain Medicine. The idea for a new study had been thought up, but that was about it.