Red Blood Cell Transfusions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

27. Clinical Indications for Cryoprecipitate And
27. CLINICAL INDICATIONS FOR CRYOPRECIPITATE AND FIBRINOGEN CONCENTRATE Cryoprecipitate is indicated in the treatment of fibrinogen deficiency or dysfibrinogenaemia.1 Fibrinogen concentrate is licenced for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenaemia and hypofibrinogenaemia,2 and is currently funded under the National Blood Agreement. Key messages y Fibrinogen is an essential component of the coagulation system, due to its role in initial platelet aggregation and formation of a stable fibrin clot.3 y The decision to transfuse cryoprecipitate or fibrinogen concentrate to an individual patient should take into account the relative risks and benefits.3 y The routine use of cryoprecipitate or fibrinogen concentrate is not advised in medical or critically ill patients.2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In the setting of major obstetric haemorrhage, early administration of cryoprecipitate or fibrinogen concentrate may be necessary.3 Clinical implications y The routine use of cryoprecipitate or fibrinogen concentrate in medical or critically ill patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of disseminated intravascular coagulopathy (MED-PP18, CC-PP7).2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In patients with critical bleeding requiring massive transfusion, suggested doses of blood components is 3-4g (CBMT-PP10)3 in adults or as per the local Massive Transfusion Protocol. -

Blood, Lymph, and Immunity Joann Colville
Blood, Lymph, and Immunity Joann Colville CHAPTER OUTLINE .»: BLOOD Function Introduction Lymphatic Structures Plasma THE IMMUNE SYSTEM Cellular Components of Blood Function Red Blood Cells Immune Reactions Platelets Immunization: Protection Against Disease White Blood Cells THE LYMPHATICSYSTEM Lymph Formation Characteristics LEARNING OBJECTIVES • List and describe the functions of blood • List the functions of the lymphatic system • Describe the composition of blood plasma • Describe the structure and functions of the lymph nodes, • Describe the characteristics of mature erythrocytes spleen, thymus, tonsils, and GALT Describe the structure of the hemoglobin molecule and • List the functions of the immune system explain the fate of hemoglobin following intravascular and • Differentiate between specific and nonspecific immune extravascular hemolysis reactions • Give the origin of thrombocytes and describe their • Differentiate between cell-mediated and humoral immunity characteristics and functions • List the components involved in cell-mediated immunity • Listthe types of leukocytes and describe the functions of each and explain the role of each • Describe the formation of lymph fluid and its circulation List and describe the classes of immunoglobulins through the lymphatic system • Differentiate between active and passive immunity BLOOD vessels of the cardiovascular system. It has three main func- tions: transportation, regulation, and defense. INTRODUCTION Blood. Say the word, and some people cringe, others faint, Function and if you believe authors Bram Stoker, Stephen King, and 1. Blood is a transport system. Christopher Moore (all authors of vampire books), others • It carries oxygen, nutrients, and other essential com- drool. But no matter what you think about blood, animals pounds to every living cell in the body. -

Transfusion Service Introduction Blood/Blood Products Requests and Turnaround Time Expectations
Transfusion Service Introduction All blood products and blood components are supplied to UnityPoint Health-Meriter Hospital by the American Red Cross Blood Services. Pathology consultation is available regarding blood and/or components and dosages. ABO and Rho(D)—specific type is used whenever possible for leuko-poor packed cell transfusions. ABO-compatible blood is used for all plasma and platelet components whenever possible. For any orders involving HLA-matched components, the patient must have been HLA typed (sent to the American Red Cross) a minimum of 48 hours prior to intended infusion of the component. HLA typing is only required once. Blood components that are thawed, pooled, washed, volume-reduced, or deglycerolized for a patient will be charged to the patient even if not transfused. The charge is done because these components may not be suitable for another patient. Examples include: Autologous or directed donations Pooled cryoprecipitate 4-hour expiration Thawed fresh frozen plasma 24-hour expiration Thawed cryoprecipitate 6-hour expiration Deglycerolized red cells 24-hour expiration Washed red cells 24-hour expiration All blood components must be completely infused within 4 hours of release from UnityPoint Health-Meriter Laboratories Blood Bank, or be infused within the expiration time. Refer to UnityPoint Health -Meriter’s Blood and Blood Products Transfusion Policy #123 for additional information located on MyMeriter. Blood/Blood Products Requests and Turnaround Time Expectations Requests from UnityPoint Health-Meriter Hospital are entered in the hospital computer system and print in the UnityPoint Health- Meriter Laboratories Blood Bank. For the comfort of the patient, it is important to coordinate collection for other tests with Blood Bank specimens. -

The Diagnosis of Paroxysmal Nocturnal Hemoglobinuria: Role of Flow Cytometry
THE DIAGNOSIS OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: ROLE OF FLOW CYTOMETRY Alberto Orfao Department of Medicine, Cancer Research Centre (IBMCC-CSIC/USAL), University of Salamanca, Salamanca, Spain Paroxysmal nocturnal hemoglobinuria (PNH) on one or multiple populations of peripheral blood is an acquired clonal disorder typically affecting red cells and/or leucocytes of PNH patients, this young adults, which involves the phosphatidylino- translating into a new diagnostic test. Because sitol glycan anchor biosynthesis class A gene of this flow cytometry became an essential tool (PIGA) coded in chromosome X, in virtually every in the diagnosis of PNH. In turn, it could be also case. The altered gene encodes for a defective pro- demonstrated that investigation of the presence of tein product, the pig-a enzyme which is involved GPI-deficient cells among circulating mature neu- in the early steps of the synthesis of glycosylphos- trophils and monocytes, increases the sensitivity phatidylinositol (GPI). This later molecule (GPI) of red blood cell screening because of the shorter acts as an anchor of a wide range of proteins to lifetime of both populations of leucocytes. Despite the cell surface. At present a large number of GPI- all the above, routine assessment of CD55 and anchor associated proteins have been described CD59 is also associated with several limitations which are expressed by one or multiple distinct due to distinct patterns of expression among dif- compartments of hematopoietic cells. The altered ferent cell populations, dim and heterogeneous PIGA gene leads to an altered GPI anchor which expression among normal individuals and the lack leads to defective expression of GPI-AP on the of experience in many labs as regards the normal cytoplasmic membrane, on the cell surface. -

Fresh Frozen Plasma in General Practice
Practical guide to using frozen and fresh frozen plasma in general practice Why every practice should keep a bag in the freezer Kit Sturgess; MA; VetMB; PhD; CertVR; DSAM; CertVC; FRCVS RCVS Recognised Specialist in Small Animal Medicine Advanced Practitioner in Veterinary Cardiology Introduction Keeping stock at reasonable levels in a practice is vital to good business management balancing the need to have a product available against the costs of keeping stock that is not used and potentially may go out of date and need to be replaced (as well as subtle small costs of space, stock taking, disposal of out-of-date product etc.). Practices need, therefore, to make decisions about preparedness for ‘what if’ scenarios and have protocols in place. The difficult question for many practices is how bizarre/uncommon should these scenarios be to warrant keeping a drug or treatment in stock or buying a specialist piece of equipment. This can only really be answered on an individual practice basis as it will depend on the type of cases seen as well as the likely ability of clients to want to pay if the treatment/investigation is expensive and the relationship with other local practices in terms of borrowing treatments or using equipment. It is also important to try and understand the value that a particular drug or treatment will have on morbidity and mortality of a particular condition and whether the patient could be referred onwards if necessary so not having calcium available if presented with a seizuring hypocalcaemic patient would be a significant issue in that patient’s care. -

Blood Banking/Transfusion Medicine Fellowship Program
DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE BLOOD BANKING/TRANSFUSION MEDICINE FELLOWSHIP PROGRAM THIS NEW ONE-YEAR ACGME-ACCREDITED FELLOWSHIP IN BLOOD BANKING/TRANSFUSION MEDICINE OFFERS STATE OF THE ART COMPREHENSIVE TRAINING IN BLOOD BANKING, COAGULATION, APHERESIS, AND HEMOTHERAPY AT THE MEMORIAL HERMANN HOSPITAL (MHH)-TEXAS MEDICAL CENTER (TMC) FOR PEDIATRICS AND ADULTS. This clinically-oriented fellowship is ideal for the candidate looking for exceptional experience in hemotherapy decision-making and coagulation consultation. We have a full spectrum of medical and surgical specialties, including a level 1 trauma center, as well as a busy solid organ transplant service (renal, liver, pancreas, cardiac, and lung). Fellows will rotate through: Our unique hemotherapy service: This innovative clinical consultative service for the Heart and Vascular Institute (HVI) allows fellows to serve as interventional blood banking consultants at the bed side as part of a multidisciplinary care team; our patients have complex bleeding and coagulopathy issues. Therapeutic apheresis service: This consultative service provide 24/7 direct patient care covering therapeutic plasmapheresis, red blood cell (RBC) exchanges, photopheresis, plateletpheresis, leukoreduction, therapeutic phlebotomies, and other related procedures on an inpatient and outpatient basis. We performed approximately over 1000 therapeutic plasma exchanges and RBC exchanges every year. Bloodbank: The MHH-TMC reference lab is one of the largest in Southeast Texas. This rotation provides extensive experience in interpreting antibody panel reports, working up transfusion reactions, investigating blood compatibility/incompatibility issues, and monitoring component usage. Gulf Coast Regional Blood Center: This rotation provides donor exposure in one of the largest community blood donation centers in the US as well as cellular therapy and immunohematology training. -

Transfusion Medicine/Blood Banking
Transfusion Medicine/Blood Banking REQUIRED rotation This is a onetime rotation and is not planned by PGY level. Objective: The objective of this rotation is to teach fellows the clinical and laboratory aspects of Transfusion Medicine and Blood Banking as it impacts hematology/oncology. Goals: The goal of this rotation is to teach fellows blood ordering practices, the difference of type and screen and type and cross matches and selection of appropriate products for transfusion. Fellows will also learn to perform, watch type and screen red cell antibodies and will learn the significance of ABO, Rh and other blood group antigen systems briefly so as to understand the significance of red cell antibodies in transfusion practices. Fellows will learn the different types of Blood components, their indications and contraindications and component modification. e.g. Irradiated and washed products, and special product request and transfusions e.g. granulocyte transfusion, CMV negative products etc. Fellows will learn and become proficient in the laboratory aspects of autoimmune hemolytic anemia, coagulopathies and bleeding disorders in relation to massive transfusions and the laboratory aspects and management of platelet refractoriness. Fellows will learn some aspects of coagulation factor replacement for factor deficiencies and inhibitors. Activities: Fellows will rotate in the Blood Bank at UMC and learn to perform, observe and interpret standard blood bank procedures such as ABO Rh typing, Antibody Screen, Antibody Identification and Direct Antiglobulin Test (DAT). Fellows will also watch platelet cross matching for platelet refractoriness. Fellows will attend and present a few didactic lectures of about 40 minutes on Blood Bank related topics as they apply to hematology/oncology like blood group antigens, Blood Component therapy, Transfusion reactions, Autoimmune hemolytic anemia, Platelet immunology and HLA as it applies to transfusion medicine. -

Chapter 06 Lecture Outline
Chapter 06 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © 2016 McGraw-Hill Education. Permission required for reproduction or display. 1 Cardiovascular System: Blood © SPL/Science Source, (inset) © Andrew Syred/Science Source 2 Points to ponder • What type of tissue is blood and what are its components? • What is found in plasma? • Name the three formed elements in blood and their functions. • How does the structure of red blood cells relate to their function? • Describe the structure and function of each white blood cell. • What are disorders of red blood cells, white blood cells, and platelets? • What do you need to know before donating blood? • What are antigens and antibodies? • How are ABO blood types determined? • What blood types are compatible for blood transfusions? • What is the Rh factor and how is this important to pregnancy? • How does the cardiovascular system interact with other systems to maintain homeostasis? 3 6.1 Blood: An Overview What are the functions of blood? • Transportation: oxygen, nutrients, wastes, carbon dioxide, and hormones • Defense: against invasion by pathogens • Regulatory functions: body temperature, water- salt balance, and body pH 4 6.1 Blood: An Overview What is the composition of blood? • Remember: blood is a fluid connective tissue. • Formed elements are produced in red bone marrow. – Red blood cells/erythrocytes (RBCs) – White blood cells/leukocytes (WBCs) – Platelets/thrombocytes 5 6.1 Blood: An Overview What is the composition of blood? • Plasma – It consists of 91% water and 9% salts (ions) and organic molecules. – Plasma proteins are the most abundant organic molecules. -
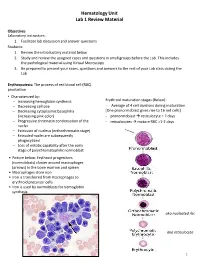
Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses. -

FDA Regulation of Blood and Blood Components in the United States
FDA Regulation of Blood and Blood Components in the United States SLIDE 1 This presentation will review the FDA Regulation of Blood and Blood Components in the U.S. SLIDE 2 The FDA Center for Biologics Evaluation and Research, or CBER, Office of Blood Research and Review, called OBRR, reviews several different types of regulatory applications with respect to blood and blood components. This includes biologics license applications, called BLAs, which represent the regulatory pathway for blood components. The BLA regulatory process also applies to biological drugs such as fractionated plasma products. OBRR also regulates in-vitro diagnostic devices used for screening of collected blood, and does so also using the BLA regulatory pathway. Review of these devices as biologic licenses allows CBER to apply a higher level of manufacturing oversight, including lot release testing and pre-licensure inspection. This regulatory pathway applies to infectious disease tests for blood screening, as well as blood grouping and phenotyping reagents. SLIDE 3 The regulatory pathway for New Drug Applications, or NDAs, is most commonly used within the Center for Drug Evaluation and Research, or CDER. Within the Office of Blood, several NDA applications are reviewed each year, mostly involving solutions used for the collection of blood, such as anticoagulants and red cell nutritive solutions. Interestingly, a blood bag that does not have a solution inside is regulated as a device. If the bag has a solution, then it is a drug-device combination product, but is regulated as a drug. SLIDE 4 OBRR reviews both Class Two and Class Three devices. Class Three devices in OBRR include diagnostic tests for HIV regulated as pre-market approvals, called PMAs. -

Donor Sex, Age and Ethnicity Impact Stored Red Blood Cell Antioxidant Metabolism Through Mechanisms in Part Explained by Glucose
Blood Transfusion SUPPLEMENTARY APPENDIX Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity Angelo D’Alessandro, 1,2,3 Xiaoyun Fu, 4 Tamir Kanias, 3,5 Julie A. Reisz, 1 Rachel Culp-Hill, 1 Yuelong Guo, 6 Mark T. Gladwin, 5 Grier Page, 6 Steve Kleinman, 7 Marion Lanteri, 8 Mars Stone, 8 Michael P. Busch, 8# and James C. Zimring 9# for the Recipi - ent Epidemiology and Donor Evaluation Study-III (REDS III) 1Department of Biochemistry and Molecular Genetics, University of Colorado Denver – Anschutz Medical Campus, Aurora, CO, USA; 2De - partment of Medicine – Division of Hematology, University of Colorado Denver – Anschutz Medical Campus, Aurora, CO, USA; 3Vitalant Re - search Institute (previously Blood Systems Research Institute), Denver, CO, USA; 4Bloodworks Northwest Research Institute, Seattle, WA, USA; 5University of Pittsburgh, Pittsburgh, PA, USA; 6RTI International, Atlanta, GA, USA; 7University of British Columbia, Victoria, Canada; 8Vi - talant Research Institute (previously Blood Systems Research Institute), San Francisco, CA, USA and 9University of Virginia, Charlotesville, VA, USA #MPB and JCZ contributed equally as co-senior authors ©2021 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol. 2020.246603 Received: January 7, 2020. Accepted: March 27, 2020. Pre-published: April 2, 2020. Correspondence: ANGELO D’ALESSANDRO - [email protected] SUPPLEMENTARY MATERIAL