Re-Evaluating Diabetic Papillopathy Using Optical Coherence Tomography and Inner Retinal Sublayer Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination
Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination Abstract We present the multimodal imaging findings of an unusual case of bilateral acquired progressive myelination of the optic disc over a 10-year follow up period, in a hyperopic adolescent patient in the absence of an underlying ocular or systemic abnormality. Myelination of the left optic disc was noted at age 7 and of the right optic disc at age 13 but no other ocular or systemic abnormalities were identified. Cross sectional OCT and en face OCT angiography confirmed the presence of myelination of the retinal nerve fiber layer and excluded other etiologic possibilities including an astrocytic hamartoma. Keywords: Optic Nerve; Optic Fiber Myelination, Acquired, Hyperopia INTRODUCTION Myelination of the retinal nerve fiber layer occurs in 1% of the population and is most frequently congenital and non-progressive. (1) Congenital cases are typically isolated but may be rarely associated with the syndrome of ipsilateral high myopia, strabismus and amblyopia (2,3), although cases have been reported in hyperopic eyes. Reports of acquired and progressive myelination associated with congenital optic nerve disorders such as Arnold-Chiari malformation, hydrocephalus, optic disc drusen, and iatrogenic causes such as optic nerve sheath fenestration, are relatively rare.(4–8) PLEASE ADJUST THE REFERENCE CITATIONS While affected patients are typically asymptomatic, retinal nerve fiber layer myelination can be rarely associated with visual loss. Even more unusual are reports of acquired and progressive myelination of the nerve fiber layer. We present the multimodal imaging findings of such a case in a healthy young patient with a 10-year follow up. -

Clinical and OCT Features of Different Types and Stages of Diabetic Optic Neuropathy P.A
Journal of Ophthalmology (Ukraine) - 2018 - Number 1 (480) Clinical and OCT features of different types and stages of diabetic optic neuropathy P.A. Bezditko 1, Dr Sc (Med), Prof.; M.A. Karliychuk 2, Cand Sc (Med) 1 Kharkiv National Medical Background: The advent of optical coherence tomography (OCT) has offered new University ; opportunities for differential diagnosis of diabetic optic neuropathy (DON). Kharkiv (Ukraine) Purpose: To identify clinical and OCT features of different types and stages of DON. 2 Higher State Educational Materials and Methods: A total of 575 patients (1150 eyes) with type 2 diabetes Establishment of Ukraine mellitus (T2DM) were included in the prospective analysis of the features of «Bukovinian State Medical optic nerve damage. For comparison, we used data from a group of 50 age- and University»; sex-matched healthy control patients. In addition to routine ocular examination, Chernivtsi (Ukraine) patients underwent ophthalmochromoscopy, OCT of the retina and optic nerve, and electrical physiological studies. E-mail: [email protected] Results: In diabetic papillopathy, anterior ischemic DON, and degenerative-, advanced-, mild-, and subclinical-stage axial DON groups, mean ganglion cell complex focal loss volume values were 21.7, 20.5, 17.3, 13.4, 10.3, and 7.5 times higher, respectively, than in controls. In diabetic papillopathy, anterior ischemic DON, and advanced-stage axial DON groups, mean peripapillary retinal thickness values were 92.2%, 84.7%, and 38.7% higher, respectively, than in controls, whereas in subclinical-, mild-, and degenerative-stage axial DON groups, the Keywords: values were 8.0%, 13.8%, and 20.5% lower, respectively, than in controls. -

Central Serous Papillopathy by Optic Nerve Head Drusen
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Central serous papillopathy by optic nerve head drusen Ana Marina Suelves1 Abstract: We report a 38-year-old man with a complaint of blurred vision in his right eye for the Ester Francés-Muñoz1 previous 5 days. He had bilateral optic disc drusen. Fluorescein angiography revealed multiple Roberto Gallego-Pinazo1 hyperfluorescent foci within temporal optic discs and temporal inferior arcade in late phase. Diamar Pardo-Lopez1 Optical coherence tomography showed bilateral peripapillary serous detachment as well as right Jose Luis Mullor2 macular detachment. This is the first reported case of a concurrent peripapillary and macular Jose Fernando Arevalo3 detachment in a patient with central serous papillopathy by optic disc drusen. Central serous papillopathy is an atypical form of central serous chorioretinopathy that should be considered Manuel Díaz-Llopis1,4,5 as a potential cause of acute loss of vision in patients with optic nerve head drusen. 1 Department of Ophthalmology, La Fe Keywords: central serous papillopathy, peripapillary central serous chorioretinopathy, optic University Hospital, Valencia, Spain; For personal use only. 2Instituto de Investigación Sanitaria, nerve head drusen, peripapillary subretinal fluid Fundación para la investigación, La Fe Hospital, Valencia, Spain; 3Retina and vitreous service, Clínica Introduction Oftalmológica Centro Caracas, Optic nerve head drusen (ONHD) are hyaline material calcificated -

Diabetic Papillopathy Diabetic Papillopathy
Teaching At the Western Ophthalmic Diabetic Papillopathy Mr Lee’s Team Diabetes; WEH May 2013 Mr Lee’s Team Diabetes; WEH May 2013 Diabetic Papillopathy Diabetic Papillopathy • Uncommon condition • 182 million people • Kanski; worldwide[X2 “Transient, mild visual in15yrs] dysfunction associated with optic disc swelling… may – 1.8 million blind be the result of small persons vessel disease ” • 2.9 million In UK • A ‘diagnosis of exclusion ’ • Controversy • Poor epidemiology; – Spectrum of AION Vs 121 case reports in Capillary vasostasis literature; incidence ~0.5% Greek myth of King Sisyphus Diabetic Papillopathy Diagnostic criterion (Slagle 2012) • Papilla [L.] a small 1. diagnosis of diabetes nipple-shaped 2. Optic disc swelling projection or (one eye or both) elevation. 3. Absence of • Pathy [G] substantial optic (pathos, “suffering”) nerve dysfunction morbid affection; 4. Normal ICP disease. 5. Lack of inflammation, infection or infiltration Optic disc swelling Blood Supply to Optic Papilla • Papilloedema – Bilateral; Normal optic nerve function; slow onset; AM headaches • Pseudopapilloedema – Bilateral; Hypermetropia; Buried Optic disc drusen • Papillitis – ~30 yrs; reduced optic nerve function; pain on mvt; sudden onset; central scotoma • Anterior ischaemic optic neuropathy (AION) – >50yrs; small disks; sudden loss of vision; altitudinal field defect • Non-Arteritic ( arteriosclerosis) • Arteritic (GCA CRP>25 ESR>50 sensitivity 90%) • Diabetic Papillopathy ? Blood Supply to Optic Papilla Blood Supply to Optic Papilla Blood Supply -

Optometric Care of the Patient with Diabetes
CANADIAN JOURNAL of OPTOMETRY | REVUE CANADIENNE D’OPTOMÉTRIE EST. 1939 VOLUME 79, SUPPLEMENT 2, 2017 CLINICAL RESEARCH 2017 CAO Clinical Practice Guideline: Optometric Care of the Patient with Diabetes Publications Mail Agreement No. 40063055 2 CANADIAN JOURNAL of OPTOMETRY | REVUE CANADIENNE D’OPTOMÉTRIE VOL. 79 SUPPLEMENT 2, 2017 CANADIAN JOURNAL of OPTOMETRY REVUE CANADIENNE D’OPTOMÉTRIE Vol 79, Supplement 2, 2017 CANADIAN JOURNAL of OPTOMETRY | REVUE CANADIENNE D’OPTOMÉTRIE Winter/Hiver 2017 EST. 1939 VOLUME 79, SUPPLEMENT 2, 2017 ISSN 0045-5075 The Canadian Journal of Optometry is the official publication of the Canadian Association of Optometrists (CAO) / La Revue canadienne CONTENTS d’optométrie est la publication officielle de l’Association canadienne des optométristes (ACO) : 234 Argyle Avenue, Ottawa ON, K2P 1B9. Phone 613 C CLINICAL RESEARCH 235-7924 / 888 263-4676, fax 613 235-2025, e-mail [email protected], website www.opto.ca. Publications Mail Registration No. 558206 / Envoi de publication 5 INTRODUCTION – Enregistrement no 558206. The Canadian Journal of Optometry / La Revue 6 ABBREVIATIONS canadienne d’optométrie (USPS#0009-364) is published four times per year. Address changes should be sent to CAO, 234 Argyle Avenue, Ottawa, 7 CLASSIFICATION, DEFINITIONS, ON K2P 1B9. RISK FACTORS DEFINITION OF DIABETES The CJO*RCO is the official publication of the CAO. However, opinions and commentaries published in 12 DIABETIC RETINAL DISEASE the CJO*RCO are not necessarily either the official opinion or policy of CAO unless specifically identified as such. Because legislation varies from province to 17 NON-RETINAL OCULAR COMPLICATIONS province, CAO advises optometrists to consult with OF DIABETES MELLITUS their provincial licensing authority before following any of the practice management advice offered in CJO*RCO. -

Neuroradiology for Ophthalmologists
Eye (2020) 34:1027–1038 https://doi.org/10.1038/s41433-019-0753-z REVIEW ARTICLE Neuroradiology for ophthalmologists 1 2 1 1,3,4,5,6,7 Bayan Al Othman ● Jared Raabe ● Ashwini Kini ● Andrew G. Lee Received: 3 June 2019 / Revised: 29 October 2019 / Accepted: 24 November 2019 / Published online: 2 January 2020 © The Author(s), under exclusive licence to The Royal College of Ophthalmologists 2020 Abstract This article will review the best approaches to neuroimaging for specific ophthalmologic conditions and discuss characteristic radiographic findings. A review of the current literature was performed to find recommendations for the best approaches and characteristic radiographic findings for various ophthalmologic conditions. Options for imaging continue to grow with modern advances in technology, and ophthalmologists should stay current on the various radiographic techniques available to them, focusing on their strengths and weaknesses for different clinical scenarios. Introduction Computed tomography (CT) 1234567890();,: 1234567890();,: Modern imaging technology continues to advance the CT imaging reconstructs a three-dimensional image made boundaries and increase the options available to physicians of many conventional x-ray images. The conventional x-ray with respect to neuroradiology. In ophthalmology, the most images are ordered in tomographic slices that have been common studies employed are computed tomography (CT) computerized so a viewer can scroll through the images, and magnetic resonance imaging (MRI). There are several analyzing sequential cross-sections [1]. Density is the pri- different types of protocols that provide unique advantages mary characteristic that determines image appearance on a and disadvantages depending on the clinical scenario. This CT scan. As with traditional x-rays, tissues appear on a grey article endeavours to review those options and discuss how scale ranging from white (i.e., hyperdense tissues such as they are best employed to evaluate a variety of specific bone) to black (i.e., hypodense materials such as air). -
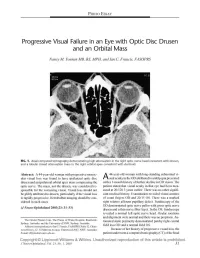
Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass
PHOTO ESSAY Progressive Visual Failure in an Eye with Optic Disc Drusen and an Orbital Mass Nancy M. Younan MB, BS, MPH, and Ian C. Francis, FASOPRS FIG. 1. Axial computed tomography demonstrating high attenuation in the right optic nerve head consistent with drusen, and a lobular mixed attenuation mass in the right orbital apex consistent with dermoid. Abstract: A 44-year-old woman with progressive monoc 44-year-old woman with long-standing subnormal vi ular visual loss was found to have ipsilateral optic disc A sual acuity in the OD attributed to amblyopia presented drusen and an ipsilateral orbital apex mass compressing the with a 1-month history of further decline in OD vision. The optic nerve. The mass, not the drusen, was considered re patient stated that visual acuity in that eye had been mea sponsible for the worsening vision. Visual loss should not sured at 20/120 3 years earlier. There was no other signifi be glibly attributed to drusen, particularly if the visual loss cant medical history. Examination revealed visual acuities is rapidly progressive. Retrobulbar imaging should be con of count fingers OD and 20/15 OS. There was a marked sidered in such cases. right relative afferent pupillary defect. Funduscopy of the OD demonstrated optic nerve pallor with gross optic nerve (JNeuro-Ophthalmol 2003;23: 31-33) drusen and a thin nerve fiber layer. In the OS, funduscopy revealed a normal left optic nerve head. Ocular rotations and alignment were normal and there was no proptosis. Au The Ocular Plastics Unit, The Prince of Wales Hospital, Randwick, tomated static perimetry demonstrated patchy right central Sydney, Australia, and the University of NSW, Sydney, Australia. -

Optic Disc Drusen, Glaucoma, Or Could It Be Both?
Title: Where are the defects coming from…Optic Disc Drusen, Glaucoma, or could it be both? Authors: Rachel Goretsky OD, Biana Gekht OD Abstract: Optic disc drusen and glaucoma cause similar retinal nerve fiber layer(RNFL) as well as visual field defects. This paper describes a patient who has both conditions and the management involved. I. Case History: 66 year old African-American male presents for dilation and imaging. Patient has a history of Glaucoma for several years; he is taking Brimonidine bid OU and Latanoprost qhs OU with reported good compliance. Medical & Ocular History: • Hypertension, gout, cholesterol and stents placed in leg and chest. Glaucoma OU. History of peripheral iridotomy OU one year ago. Medications:Amplodipine, clopidogrel, allopurinol II. Pertinent findings: • Maximum pressure history is 17 OD, 19 OS, current visit 16 OD, 19 OS • Anterior chamber : Grade 2 Van Herick Angles OU • Iris: Peripheral Iridotomy, patent at 12 o’clock OD,OS • Optic disc: 0.3r OD, OS small nerves, mild blurry margins indicative of disc drusen • Macula: diffuse epiretinal membrane OU • Pachymetry : 556 OD/ 566 OS • Gonioscopy: open to trabecular meshwork (TM) superiorly, otherwise no structures visible OD, open to TM inferiorly, otherwise no structures visible OS • Spectralis Optical Coherence Tomography(OCT): optic disc drusen with small cups and disc area OU • Visual field exhibited no defects OD, inferior nasal defects OS. III. Differential Diagnosis: • Papilledema, tilted discs, pseudotumor cerebri, myelinated nerve fiber layer IV. Diagnosis and Discussion Optic nerve head drusen are hyaline deposits made of calcium phosphate as well as amino acids, among other materials. -
Optic Nerve on Sheath:
3/16/2018 < Optic nerve axons of retinal ganglion cells 1.2 million nerve fibers . ON sheath: continuous with the meninges dura、arachnoid and pia mater 1 3/16/2018 optic nerve functions 1.Visual Acuity 2.Color Vision 3.Pupil 4.Contrast sensitivity Ancillary Tests 1.Visual Field 2.Neuro-imaging 3.OCT 4.VEP Etiology:Optic nerve diseases 1.inflammation:optic neuritis 2 . ischemic optic neuropathy 3-Compression 4-Granuloma & infiltration 5-Hereditary 6-Toxic 7-Irradiation 8- Trauma . 2 3/16/2018 Optic Neuritis 3-Neuroretinitis 1-Retrobulbar neuritis 2-Papillitis Papillitis optic disc is normal hyperemia and edema with macular star . in adults common in children. least common type with multiple sclerosis. viral infections Rapid unilateral loss of vision RAPD Loss of color vision Pain in moving the eye Swollen disc with or without peripapillary flame-shaped hemorrhages. 3 3/16/2018 Fig optic neuritis Centrocecal scotoma Bilateral optic neuritis Bilateral Central scotoma 4 3/16/2018 Bilateral hemianopsia MRI demyelinating lesions multiple sclerosis Neuromylitis Optica VF showed non- central scotoma altitudinal VF an ischemic mechanism play a role in ON in NMO patients 5 3/16/2018 Optic Neuritis Follow Up Diffuse and central loss in the affected eye at baseline Follow Up : nerve fiber bundle defects were the predominant localized abnormalities in both the affected and fellow eyes physicians evaluate the characteristics of optic neuritis and other optic neuropathies in the future Ischemic optic neuropathy ( ION) 6 3/16/2018 Anterior ischemic optic neuropathy ( AION ) Arteritic Non- Arteritic GCA - vasculitis hypoperfusion of ONH transient visual loss, temporal sudden painless loss of vision pain, jaw pain, fatigue, weight loss hyperemic disc Pale disc Sectorial, diffuse edema optic disc edema splinter hemorrhages of a chalky white color Subsequent optic atrophy Nonarteritic ischemic optic neuropathy visual field altitudinal field defect 7 3/16/2018 NAION sudden, painless visual loss OS shows AION . -

Diabetic Papillopathy 213
ch. 22 / Diabetic Papillopathy 213 chDR.RUPNATHJI( DR.RUPAK22 NATH ) Diabetic Papillopathy Or Non-Arteritic Ischemic Optic Neuropathy That Happens to Occur in Diabetics. Or Whatever... 214 Diabetic Retinopathy This is an entity that does not quite fi t into the categories of non-proliferative or proliferative disease. It is traditionally considered a relatively mild problem, but on occasion, it can be a real pain because it may create both a diagnostic and therapeutic challenge. It can also be easy to miss, especially if the patient has a lot of widespread macular edema that masks subtle swelling on the temporal aspect of the optic nerve. You do not want to miss it, though, because it adds a potential wild card to the patient’s visual prognosis, and it is far better to recog- nize the problem and educate the patient than to be surprised. This is particu- larly true if a patient has vision loss due to papillopathy at some point after a laser; it is hard to retroactively convince the patient that the laser did not cause the problem. Diabetic papillopathy was originally described in juvenile (Type 1) diabetic pa- tients, but since then it has been described in older, Type 2 patients as well. The problem is most likely related to capillary damage that results in chronic isch- emia and secondary nerve swelling, which, for reasons known only to the gods of diabetes, does not tip the nerve into full-blown ischemic optic neuropathy (usually). Perhaps the nerve doesn’t lapse into full-fl edged visual loss because the vascular system just isn’t that bad—something which may go along with it occurring in younger patients. -

Mizuo-Nakamura Phenomenon in Oguchi Disease Due to A
Correspondence 1098 lattice-like degeneration,4 non-neovascular vitreous haemorrhage,5 and myopia.4 Occasionally elevated blood vessels have been demonstrated secondary to vitreous traction.6 We postulate that in this case separation of intact retinal vascular arcades from the retina occurred because of antero–posterior mechanical forces on already dragged retinal vessels under tension when PVD occurred. Kingham also advocated conservative management for such cases. Conflict of interest The authors declare no conflict of interest. References 1 Shaikh S, Trese MT. New Insights into progressive visual loss in adult retinopathy of prematurity. Arch Ophthalmol 2004; 122(3): 404–406. 2 Tasman W. Vitreoretinal changes in cicatrical retrolental fibroplasias. Trans Am Ophthalmol soc 1970; 68: 548–594. 3 Kingham JD. Acute retrolental fibroplasia. II. Treatment by cryosurgery. Arch Ophthalmol 1978; 96(11): 2049–2053. 4 Smith BT, Tasman WS, Young TL, Wilson ME, Raab EL, Paysse EA et al. Retinopathy of prematurity: late complications in the baby boomer generation (1946–1964). Trans Am Ophthalmol Soc 2005; 103: 225–234. 5 Quiram PA, Capone Jr A. Adult ROP: late complications of retinopathy of prematurity. Retinal Physician 2007; 4(5): 25–28. 6 Tasman W, Brown GC. Progressive visual loss in adults with retinopathy of prematurity (ROP). Trans Am Ophthalmol Soc 1988; 86: 367–379. S Tarafdar, EE Obi and JR Murdoch Tennent Institute of Ophthalmology, Gartnavel Figure 1 (a) A nasally dragged disc and retinal vessels. (b) Intact General Hospital, Glasgow, Scotland, UK avulsed retinal vessels floating freely in the vitreous cavity. E-mail: [email protected] avulsed retinal vessels floating freely in the vitreous Eye (2011) 25, 1097–1098; doi:10.1038/eye.2011.86; cavity was present (Figure 1b).