Congenital Anomalies of the Optic Nerve
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reorganisation of the Visual Cortex in Callosal Agenesis and Colpocephaly
Journal of Clinical Neuroscience (2000) 7(1), 13–15 © 2000 Harcourt Publishers Ltd DOI: 10.1054/ jocn.1998.0105, available online at http://www.idealibrary.com on Clinical study Reorganisation of the visual cortex in callosal agenesis and colpocephaly Richard G. Bittar1,2 MB BS, Alain Ptito1 PHD, Serge O. Dumoulin1, Frederick Andermann1 MD FRCP(C), David C. Reutens1 MD FRACP 1Montreal Neurological Institute and Hospital and Department of Neurology and Neurosurgery, McGill University, Montreal, Canada 2Department of Anatomy and Histology, University of Sydney, Australia Summary Structural defects involving eloquent regions of the cerebral cortex may be accompanied by abnormal localisation of function. Using functional magnetic resonance imaging (fMRI), we studied the organisation of the visual cortex in a patient with callosal agenesis and colpocephaly, whose visual acuity and binocular visual fields were normal. The stimulus used was a moving grating confined to one hemifield, on a background of moving dots. In addition to activation patterns elicited by stimulation of each hemifield in the patient, the activation pattern was compared to that seen in six normal volunteers. fMRI demonstrated large scale reorganisation of visual cortical areas in the left hemisphere, and fewer activation foci were observed in both occipital lobes when compared with normal subjects. © 2000 Harcourt Publishers Ltd Keywords: functional magnetic resonance imaging, vision, colpocephaly, callosal agenesis, reorganisation INTRODUCTION showed interictal slow wave activity throughout the right hemi- sphere and seizures with onset in the right temporo-frontal region. An exciting application of non-invasive functional brain mapping Ictal Tc99m HMPAO single photon emission computed tomography techniques such as functional magnetic resonance imaging (fMRI) (SPECT) revealed a diffuse increase in perfusion within the right is the study of cortical sensory and motor reorganisation following cerebral hemisphere. -

Colpocephaly Diagnosed in a Neurologically Normal Adult in the Emergency Department
CASE REPORT Colpocephaly Diagnosed in a Neurologically Normal Adult in the Emergency Department Christopher Parker, DO University of Illinois College of Medicine, Department of Emergency Medicine, Wesley Eilbert, MD Chicago, Illinois Timothy Meehan, MD Christopher Colbert, DO Section Editor: Rick A. McPheeters, DO Submission history: Submitted July 25, 2019; Revision received September 18, 2019; Accepted September 26, 2019 Electronically published October 21, 2019 Full text available through open access at http://escholarship.org/uc/uciem_cpcem DOI: 10.5811/cpcem.2019.9.44646 Colpocephaly is a form of congenital ventriculomegaly characterized by enlarged occipital horns of the lateral ventricles with associated neurologic abnormalities. The diagnosis of colpocephaly is typically made in infancy. Its diagnosis in adulthood without associated clinical symptoms is exceptionally rare. We report a case of colpocephaly diagnosed incidentally in an adult without neurologic abnormalities in the emergency department. To our knowledge, this is only the ninth reported case in an asymptomatic adult and the first to be described in the emergency medicine literature. [Clin Pract Cases Emerg Med. 2019;3(4):421–424.] INTRODUCTION been treated at four different EDs in the two weeks prior to Colpocephaly is a rare form of congenital presentation for the headaches, but no imaging studies had ventriculomegaly often associated with partial or been performed. The patient had no psychiatric history. His complete agenesis of the corpus callosum. Diagnosis is highest level of education was a high school diploma, and typically made in infancy due to associated neurological he was unemployed. and neurodevelopmental disorders.1,2 Initial discovery On arrival, the patient was afebrile with pulse, blood in adulthood is exceedingly rare.3-9 When identified pressure, and respiratory rate all within the normal range. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Bilateral Posterior Periventricular Nodular Heterotopia: a Recognizable Cortical Malformation with a Spectrum of Associated Brain Abnormalities
ORIGINAL RESEARCH PEDIATRICS Bilateral Posterior Periventricular Nodular Heterotopia: A Recognizable Cortical Malformation with a Spectrum of Associated Brain Abnormalities S.A. Mandelstam, R.J. Leventer, A. Sandow, G. McGillivray, M. van Kogelenberg, R. Guerrini, S. Robertson, S.F. Berkovic, G.D. Jackson, and I.E. Scheffer ABSTRACT BACKGROUND AND PURPOSE: Bilateral posterior PNH is a distinctive complex malformation with imaging features distinguishing it from classic bilateral PNH associated with FLNA mutations. The purpose of this study was to define the imaging features of posterior bilateral periventricular nodular heterotopia and to determine whether associated brain malformations suggest specific subcategories. MATERIALS AND METHODS: We identified a cohort of 50 patients (31 females; mean age, 13 years) with bilateral posterior PNH and systematically reviewed and documented associated MR imaging abnormalities. Patients were negative for mutations of FLNA. RESULTS: Nodules were often noncontiguous (n ϭ 28) and asymmetric (n ϭ 31). All except 1 patient showed associated developmental brain abnormalities involving a spectrum of posterior structures. A range of posterior fossa abnormalities affected the cerebellum, including cerebellar malformations and posterior fossa cysts (n ϭ 38). Corpus callosum abnormalities (n ϭ 40) ranged from mild dysplasia to agenesis. Posterior white matter volume was decreased (n ϭ 22), and colpocephaly was frequent (n ϭ 26). Most (n ϭ 40) had associated cortical abnormalities ranging from minor to major (polymicrogyria), typically located in the cortex overlying the PNH. Abnormal Sylvian fissure morphology was common (n ϭ 27), and hippocampal abnormalities were frequent (n ϭ 37). Four family cases were identified—2 with concordant malformation patterns and 2 with discordant malformation patterns. -

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Genetic and Developmental Basis of Renal Coloboma (Papillorenal) Syndrome
Perspective Genetic and developmental basis of renal coloboma (papillorenal) syndrome Expert Rev. Ophthalmol. 4(2), 135–xxx (2009) Lisa A Schimmenti Renal coloboma syndrome, also known as papillorenal syndrome, is characterized by optic Department of Pediatrics and nerve anomalies and kidney hypodysplasia. Autosomal dominant mutations in the gene Ophthalmology, University of encoding the paired box transcription factor PAX2 can be identified in nearly half of all Minnesota Medical School, 420 patients with this phenotype. The primary ophthalmologic findings include congenital central Delaware Street Southeast, retinal vasculature absence associated with abnormalities in retinal blood vessel patterning MMC 730, Minnesota, and deeply excavated optic discs. Other published findings include optic nerve hypoplasia, MN 55455, USA optic nerve cyst, optic nerve pits, retinal coloboma, microphthalmia and scleral staphyloma. Tel.: +1 612 624 5613 Visual acuity ranges from normal to severe impairment. Up to one third of affected patients Fax: +1 612 626 2993 will develop end-stage renal disease. Mouse and zebrafish withPax2/pax2a mutations provide [email protected] developmentally based explanations for the observed phenotypic observations in affected patients. KEYWORDS: optic nerve coloboma • optic nerve dysplasia • papillorenal syndrome • PAX2 • renal coloboma syndrome The clinical presentation of optic nerve anomalies total of 10 years later, Weaver et al. reported a case associated with renal hypodysplasia should alert the of two brothers, both with optic nerve colobomas, clinician to the possibility that a patient may have interstitial nephritis and renal failure [3]. Weaver renal coloboma syndrome, a condition also known noted the similarity of the optic nerve colobomas as papillorenal syndrome (OMIM#120330). The in his patients with the ‘morning glory anomaly’, optic nerve findings could be described as a ‘dys- previously reported by Karcher et al. -

Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination
Bilateral Acquired Progressive Retinal Nerve Fiber Layer Myelination Abstract We present the multimodal imaging findings of an unusual case of bilateral acquired progressive myelination of the optic disc over a 10-year follow up period, in a hyperopic adolescent patient in the absence of an underlying ocular or systemic abnormality. Myelination of the left optic disc was noted at age 7 and of the right optic disc at age 13 but no other ocular or systemic abnormalities were identified. Cross sectional OCT and en face OCT angiography confirmed the presence of myelination of the retinal nerve fiber layer and excluded other etiologic possibilities including an astrocytic hamartoma. Keywords: Optic Nerve; Optic Fiber Myelination, Acquired, Hyperopia INTRODUCTION Myelination of the retinal nerve fiber layer occurs in 1% of the population and is most frequently congenital and non-progressive. (1) Congenital cases are typically isolated but may be rarely associated with the syndrome of ipsilateral high myopia, strabismus and amblyopia (2,3), although cases have been reported in hyperopic eyes. Reports of acquired and progressive myelination associated with congenital optic nerve disorders such as Arnold-Chiari malformation, hydrocephalus, optic disc drusen, and iatrogenic causes such as optic nerve sheath fenestration, are relatively rare.(4–8) PLEASE ADJUST THE REFERENCE CITATIONS While affected patients are typically asymptomatic, retinal nerve fiber layer myelination can be rarely associated with visual loss. Even more unusual are reports of acquired and progressive myelination of the nerve fiber layer. We present the multimodal imaging findings of such a case in a healthy young patient with a 10-year follow up. -

Ophthalmology
Ophthalmology Information for health professionals MEDICAL GENETIC TESTING FOR OPHTHALMOLOGY Recent technologies, in particularly Next Generation Sequencing (NGS), allows fast, accurate and valuable diagnostic tests. For Ophthalmology, CGC Genetics has an extensive list of medical genetic tests with clinical integration of results by our Medical Geneticists. 1. EXOME SEQUENCING: Exome Sequencing is a very efficient strategy to study most exons of a patient’s genome, unraveling mutations associated with specific disorders or phenotypes. With this diagnostic strategy, patients can be studied with a significantly reduced turnaround time and cost. CGC Genetics has available 2 options for Exome Sequencing: • Whole Exome Sequencing (WES), which analyzes the entire exome (about 20 000 genes); • Disease Exome by CGC Genetics, which analyzes about 6 000 clinically-relevant genes. Any of these can be performed in the index case or in a Trio. 2. NGS PANELS For NGS panels, several genes associated with the same phenotype are simultaneously sequenced. These panels provide increased diagnostic capability with a significantly reduced turnaround time and cost. CGC Genetics has several NGS panels for Ophthalmology that are constantly updated (www.cgcgenetics.com). Any gene studied in exome or NGS panel can also be individually sequenced and analyzed for deletion/duplication events. 3. EXPERTISE IN MEDICAL GENETICS CGC Genetics has Medical Geneticists specialized in genetic counseling for ophthalmological diseases who may advice in choosing the most appropriate -

CONGENITAL ABNORMALITIES of the CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles Ffrench-Constant *I3
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_1.i3 on 1 March 2003. Downloaded from CONGENITAL ABNORMALITIES OF THE CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles ffrench-Constant *i3 J Neurol Neurosurg Psychiatry 2003;74(Suppl I):i3–i8 dvances in genetics and molecular biology have led to a better understanding of the control of central nervous system (CNS) development. It is possible to classify CNS abnormalities Aaccording to the developmental stages at which they occur, as is shown below. The careful assessment of patients with these abnormalities is important in order to provide an accurate prog- nosis and genetic counselling. c NORMAL DEVELOPMENT OF THE CNS Before we review the various abnormalities that can affect the CNS, a brief overview of the normal development of the CNS is appropriate. c Induction—After development of the three cell layers of the early embryo (ectoderm, mesoderm, and endoderm), the underlying mesoderm (the “inducer”) sends signals to a region of the ecto- derm (the “induced tissue”), instructing it to develop into neural tissue. c Neural tube formation—The neural ectoderm folds to form a tube, which runs for most of the length of the embryo. c Regionalisation and specification—Specification of different regions and individual cells within the neural tube occurs in both the rostral/caudal and dorsal/ventral axis. The three basic regions of copyright. the CNS (forebrain, midbrain, and hindbrain) develop at the rostral end of the tube, with the spinal cord more caudally. Within the developing spinal cord specification of the different popu- lations of neural precursors (neural crest, sensory neurones, interneurones, glial cells, and motor neurones) is observed in progressively more ventral locations. -

Chiari Type II Malformation: Past, Present, and Future
Neurosurg Focus 16 (2):Article 5, 2004, Click here to return to Table of Contents Chiari Type II malformation: past, present, and future KEVIN L. STEVENSON, M.D. Children’s Healthcare of Atlanta, Atlanta, Georgia Object. The Chiari Type II malformation (CM II) is a unique hindbrain herniation found only in patients with myelomeningocele and is the leading cause of death in these individuals younger than 2 years of age. Several theories exist as to its embryological evolution and recently new theories are emerging as to its treatment and possible preven- tion. A thorough understanding of the embryology, anatomy, symptomatology, and surgical treatment is necessary to care optimally for children with myelomeningocele and prevent significant morbidity and mortality. Methods. A review of the literature was used to summarize the clinically pertinent features of the CM II, with par- ticular attention to pitfalls in diagnosis and surgical treatment. Conclusions. Any child with CM II can present as a neurosurgical emergency. Expeditious and knowledgeable eval- uation and prompt surgical decompression of the hindbrain can prevent serious morbidity and mortality in the patient with myelomeningocele, especially those younger than 2 years old. Symptomatic CM II in the older child often pre- sents with more subtle findings but rarely in acute crisis. Understanding of CM II continues to change as innovative techniques are applied to this challenging patient population. KEY WORDS • Chiari Type II malformation • myelomeningocele • pediatric The CM II is uniquely associated with myelomeningo- four distinct forms of the malformation, including the cele and is found only in this population. Originally de- Type II malformation that he found exclusively in patients scribed by Hans Chiari in 1891, symptomatic CM II ac- with myelomeningocele. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -
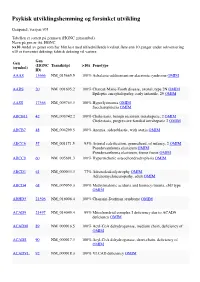
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD