New York State Immunization Requirements for School Entrance/Attendance1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Frequently Asked Questions About Measles Immunizations
Immunization Unit Assessment, Compliance, and Evaluation Group Measles FAQs Q: When did the vaccine for measles become available? A: The first measles vaccines were available in 1963, and were replaced with a much better vaccine in 1968. Q: Do people who received MMR in the 1960s need to have their dose repeated? A: Not necessarily. People who have documentation of receiving a live measles vaccine in the 1960s do not need to be revaccinated. People who were vaccinated with a killed or inactivated measles vaccine, or vaccine of unknown type, should be revaccinated with at least 1 dose of MMR. Q: What kind of vaccine is it? A: The vaccines available in the United States that protect against measles are the MMR and MMRV, which protects against measles, mumps, and rubella or measles, mumps, rubella, and varicella (chickenpox). They are both live attenuated, or weakened, vaccines. Q: Which vaccine can I get, the MMR or the MMRV? A: The MMRV is only approved for people aged 12 months – 12 years. The MMR is approved for anyone 6 months of age and older, but it is routinely recommended for use in people 12 months of age and older. Any dose of MMR received before 12 months of age is not considered valid and should be repeated in 4 weeks or at age 12 months, whichever is later. Q: How is the vaccine given? A: The vaccine is administered in the fatty layer of tissue underneath the skin. Q: Is the measles shot just a one-time dose? A: The measles vaccine is very effective. -

Measles: Chapter 7.1 Chapter 7: Measles Paul A
VPD Surveillance Manual 7 Measles: Chapter 7.1 Chapter 7: Measles Paul A. Gastanaduy, MD, MPH; Susan B. Redd; Nakia S. Clemmons, MPH; Adria D. Lee, MSPH; Carole J. Hickman, PhD; Paul A. Rota, PhD; Manisha Patel, MD, MS I. Disease Description Measles is an acute viral illness caused by a virus in the family paramyxovirus, genus Morbillivirus. Measles is characterized by a prodrome of fever (as high as 105°F) and malaise, cough, coryza, and conjunctivitis, followed by a maculopapular rash.1 The rash spreads from head to trunk to lower extremities. Measles is usually a mild or moderately severe illness. However, measles can result in complications such as pneumonia, encephalitis, and death. Approximately one case of encephalitis2 and two to three deaths may occur for every 1,000 reported measles cases.3 One rare long-term sequelae of measles virus infection is subacute sclerosing panencephalitis (SSPE), a fatal disease of the central nervous system that generally develops 7–10 years after infection. Among persons who contracted measles during the resurgence in the United States (U.S.) in 1989–1991, the risk of SSPE was estimated to be 7–11 cases/100,000 cases of measles.4 The risk of developing SSPE may be higher when measles occurs prior to the second year of life.4 The average incubation period for measles is 11–12 days,5 and the average interval between exposure and rash onset is 14 days, with a range of 7–21 days.1, 6 Persons with measles are usually considered infectious from four days before until four days after onset of rash with the rash onset being considered as day zero. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

Measles, Mumps and Rubella Gone but Not Forgotten
Measles, mumps and rubella Gone but not forgotten David Isaacs Measles z Highly infectious human disease z 1 in 15 cases develop complications – otitis me dia, pneumoni a, encephalitis, SSPE z Pre vaccine epidemiology – 2 - 5 year cycle of epidemics – most common in 5 - 9 year olds – world wide 7 - 8 million deaths annually Measles z 99.9% of unimmunised children get measles z 1 in 5,000 to 10,000 cases die z Mortality : 888,000 deaths in 1998 (more than breast cancer, violence) z Morbidity : encephalitis (0.1%), convulsions ((%),p0.5%), pneumonia (1-7%), otitis media (5-9%) Measles: a global perspective z Vaccine available for over 40 years z Still caused 197,000 estimated deaths in 2007 – leading vaccine preventable killer of children z Highest disease incidence in Africa z Most deaths (98%) are in poorest countries – low vaccination coverage, high case fatality ratio Measles in Australia 1200 Notifications 60 Hospitalisations 50 1000 40 2001 – young 30 adults program Number 20 800 10 rr 0 Jan Jan Jan Jan Jan Jan Jan Jan Jan 600 2000 2001 2002 2003 2004 2005 2006 2007 2008 Month Numbe 400 200 0 Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 Month Nov 1993 – 2nd dose Jul 1998 – 2nd dose moved to 4-5 yo introduced for 10-16 yo Measles Control Campaign conducted Measles elimination in Australia: 1 The Meas les Con tro l campa ign (MCC) – July-December 1998 – mass vaccination 1.7 million primary school children (96% vaccinated) – reminder letter -

AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Kids and COVID 19 Vaccination
Kids and COVID 19 Vaccination Q: What COVID vaccine is available for kids right now? A: Pfizer-BioNTech mRNA vaccine was initially approved for individuals to as young as age 16 years old and was recently approved for kids as young as 12 years of age. This is a two shot series, given 3 weeks apart. The dose is the same as that given to adults. Q: When will younger kids be able to get vaccinated? A: Pfizer is currently conducting studies on kids as young as 6 months of age. Moderna is in the process of submitting data on their studies for ages 12-18 yrs and have started the process for studies in younger kids. Many reputable sources suggest that kids as young as 6yrs of age may be eligible for vaccination this fall. Keep checking our website for more information on timeline and details for vaccination in younger kids and infants. Q: How do I get my child vaccinated? A: In North Idaho, there are many locations that offer the Pfizer vaccine. Many local pharmacies have the Pfizer vaccine available (check online or in person to ensure they have Pfizer brand available for those under age 18 yrs). The local Walgreen’s pharmacies are offering Pfizer vaccine and appointments can be scheduled at www.walgreens.com. Other option: www.panhandlehealth.org. This site will allow you to make appointments at the Kootenai county fairgrounds site as well as other sites in adjacent counties. We are not yet offering COVID vaccination at Coeur d’Alene Pediatrics, but hope to be able to do so in the future. -

Statement of David D
THE AMERICAN ASSOCIATION OF IMMUNOLOGISTS Statement of David D. Chaplin, M.D., Ph.D., Chair of the Committee on Public Affairs of The American Association of Immunologists (AAI), Regarding the Importance of Vaccines March 5, 2019 Each year, seasonal outbreaks of influenza cause an estimated 9.3 million to 49 million illnesses, 140,000 to 960,000 hospitalizations, and 12,000 to 79,000 deaths in the United States.1 Other serious infectious diseases also cause occasional outbreaks, often with devastating consequences. A growing outbreak of measles – a dangerous, highly contagious, and potentially lethal disease – is currently spreading in 10 states. It is easily transmitted through the air by coughing and sneezing and by contact with contaminated surfaces. There is no anti-viral treatment for measles. Children younger than five and adults over 20 are at the highest risk of serious complications, including blindness, swelling of the brain, and severe pneumonia.2 Those with compromised immune systems or underlying health conditions who cannot get vaccinated are also at significant risk of complications and death. The spread of many diseases can be limited, or prevented, by available vaccines. For example, efficacy of the existing measles vaccine [measles, mumps, rubella, or “MMR”] is about 97%,3 and its widespread use could prevent measles entirely.4 In other instances, such as with the influenza vaccine, immunization can also lessen the severity of disease, reducing the number of hospitalizations and saving lives.5 AAI is concerned that some parents are choosing not to have their children vaccinated; among the reasons cited is their uncertainty about vaccine safety. -
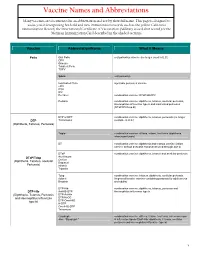
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand .................................................................... -

Vaccines & Immunizations
v ¡;ccines: Vac-GenIhat Would Happen If We Stopped Vaccinations Page 1 of6 Vaccines & Immunizations Basics and Common Questions: What Woulcj .l-aepen.lr'!e§tC?peecj"ya~~inati9r,s? In the U.S., vaccination programs have eliminated or significantly reduced many vaccine-preventable diseases. However, these diseases still exist and can once again become common-and deadly-if vaccination coverage does not continue at high levels. On this page (Figures and Statistics Updated 2003): Introduction Measles Polio Type b (Hib) Meningitis Hepatitis B Pertussis (Whooping Cough) Pneumococcal Rubella (German Measles) Varicella (Chickenpox) Diphtheria Tetanus (Lockjaw) Mumps I ntrod uction In the U.S., vaccines have reduced or eliminated many infectious diseases that once routinely killed or harmed many infants, children, and adults. However, the viruses and bacteria that cause vaccine-preventable disease and death still exist and can be passed on to people who are not protected by vaccines. Vaccine-preventable diseases have many social and economic costs: sick children miss school and can cause parents to lose time from work. These diseases also result in doctor's visits, hospitalizations, and even premature deaths. OTôP Polio Stopping vaccination against polio will leave people susceptible to infection with the polio virus. Polio virus causes acute paralysis that can lead to permanent physical disability and even death. Before polio vaccine was available, 13,000 to 20,000 cases of paralytic polio were reported each year in the United States. These annual epidemics of polio often left thousands of victims--mostly children--in braces, crutches, wheelchairs, and iron lungs. The effects were life-long.