Vaccines to Children Protective Effect and Adverse Events
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measles, Mumps and Rubella Gone but Not Forgotten
Measles, mumps and rubella Gone but not forgotten David Isaacs Measles z Highly infectious human disease z 1 in 15 cases develop complications – otitis me dia, pneumoni a, encephalitis, SSPE z Pre vaccine epidemiology – 2 - 5 year cycle of epidemics – most common in 5 - 9 year olds – world wide 7 - 8 million deaths annually Measles z 99.9% of unimmunised children get measles z 1 in 5,000 to 10,000 cases die z Mortality : 888,000 deaths in 1998 (more than breast cancer, violence) z Morbidity : encephalitis (0.1%), convulsions ((%),p0.5%), pneumonia (1-7%), otitis media (5-9%) Measles: a global perspective z Vaccine available for over 40 years z Still caused 197,000 estimated deaths in 2007 – leading vaccine preventable killer of children z Highest disease incidence in Africa z Most deaths (98%) are in poorest countries – low vaccination coverage, high case fatality ratio Measles in Australia 1200 Notifications 60 Hospitalisations 50 1000 40 2001 – young 30 adults program Number 20 800 10 rr 0 Jan Jan Jan Jan Jan Jan Jan Jan Jan 600 2000 2001 2002 2003 2004 2005 2006 2007 2008 Month Numbe 400 200 0 Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan Jan 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 Month Nov 1993 – 2nd dose Jul 1998 – 2nd dose moved to 4-5 yo introduced for 10-16 yo Measles Control Campaign conducted Measles elimination in Australia: 1 The Meas les Con tro l campa ign (MCC) – July-December 1998 – mass vaccination 1.7 million primary school children (96% vaccinated) – reminder letter -

AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -
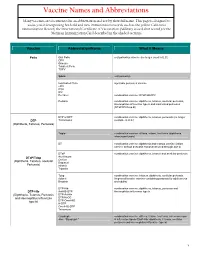
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

Vaccines & Immunizations
v ¡;ccines: Vac-GenIhat Would Happen If We Stopped Vaccinations Page 1 of6 Vaccines & Immunizations Basics and Common Questions: What Woulcj .l-aepen.lr'!e§tC?peecj"ya~~inati9r,s? In the U.S., vaccination programs have eliminated or significantly reduced many vaccine-preventable diseases. However, these diseases still exist and can once again become common-and deadly-if vaccination coverage does not continue at high levels. On this page (Figures and Statistics Updated 2003): Introduction Measles Polio Type b (Hib) Meningitis Hepatitis B Pertussis (Whooping Cough) Pneumococcal Rubella (German Measles) Varicella (Chickenpox) Diphtheria Tetanus (Lockjaw) Mumps I ntrod uction In the U.S., vaccines have reduced or eliminated many infectious diseases that once routinely killed or harmed many infants, children, and adults. However, the viruses and bacteria that cause vaccine-preventable disease and death still exist and can be passed on to people who are not protected by vaccines. Vaccine-preventable diseases have many social and economic costs: sick children miss school and can cause parents to lose time from work. These diseases also result in doctor's visits, hospitalizations, and even premature deaths. OTôP Polio Stopping vaccination against polio will leave people susceptible to infection with the polio virus. Polio virus causes acute paralysis that can lead to permanent physical disability and even death. Before polio vaccine was available, 13,000 to 20,000 cases of paralytic polio were reported each year in the United States. These annual epidemics of polio often left thousands of victims--mostly children--in braces, crutches, wheelchairs, and iron lungs. The effects were life-long. -

Recommendations for Immunizations and TB Testing for Health Science Students
Recommendations for Immunizations and TB Testing for Health Science Students Overview Influenza: 1 dose of inactivated Influenza vaccine yearly. Hepatitis B: 3-dose series of hepatitis B vaccine given at 0, 1 and 6 months AND documented quantitative hepatitis B surface antibody titer consistent with immunity after the appropriate vaccine series. Measles/Mumps/Rubella (MMR): 2 doses of MMR vaccine at least 28 days apart after 12 months of age OR 2 doses of measles and 2 doses of Mumps at least 28 days apart after 12 months of age and one dose of rubella after 12 months of age OR laboratory proof of immunity to measles/mumps/rubella. Tetanus/Diphtheria/Pertussis: In addition to primary series, all Health Care Personnel (HCP) should receive 1 dose of Tdap and have documentation of a Td or Tdap within the past 10 years. Tuberculosis Testing: The CDC recommends initial base line testing with a 2-step TB skin test or a blood test for TB infection. Subsequent annual or serial screening is determined by state regulations or risk assessment. Varicella: 2 doses of varicella vaccine given at least 4 weeks apart OR laboratory proof of immunity for those with a history of disease. If titer is negative or equivocal, give 2-dose varicella vaccine series. Do not repeat titer after series completion. Hepatitis B: Students must have a series of 3 hepatitis B vaccines AND a positive (≥10 mIU/mL) serological quantitative Hepatitis B surface antibody titer (anti-HBs or HBsAb) that was performed at least 1-2 months after the 3rd dose of hepatitis B vaccine. -

Addressing Parents' Concerns: Do Multiple Vaccines Overwhelm Or Weaken the Infant's Immune System? Paul A
Addressing Parents’ Concerns: Do Multiple Vaccines Overwhelm or Weaken the Infant’s Immune System? Paul A. Offit, MD*; Jessica Quarles‡; Michael A. Gerber, MD§; Charles J. Hackett, PhDʈ; Edgar K. Marcuse, MD¶; Tobias R. Kollman, MD#; Bruce G. Gellin, MD**; and Sarah Landry‡ ABSTRACT. Recent surveys found that an increasing many as 20 shots by 2 years of age (Table 1). The number of parents are concerned that infants receive too increased number of vaccines given to children and many vaccines. Implicit in this concern is that the infant’s the increased percentage of children receiving vac- immune system is inadequately developed to handle vac- cines have resulted in a dramatic decrease in the cines safely or that multiple vaccines may overwhelm the number of vaccine-preventable diseases. Most young immune system. In this review, we will examine the parents today have never seen many of the diseases following: 1) the ontogeny of the active immune response and the ability of neonates and young infants to respond that vaccines prevent. As a possible consequence of to vaccines; 2) the theoretic capacity of an infant’s im- these trends, recent national surveys found that 23% mune system; 3) data that demonstrate that mild or mod- of parents questioned the number of shots recom- erate illness does not interfere with an infant’s ability to mended for their children,1 and 25% were concerned generate protective immune responses to vaccines; 4) that vaccines might weaken the immune system.1 how infants respond to vaccines given in combination Because most parents receive information and rec- compared with the same vaccines given separately; 5) ommendations about vaccines from their doctors,2 data showing that vaccinated children are not more likely and because these recommendations carry substan- to develop infections with other pathogens than unvac- tial weight with parents,3,4 providers must be knowl- cinated children; and 6) the fact that infants actually edgeable when addressing parents’ concerns. -

Vaccine Preventable Diseases 1 Surveillancewho Vaccine-Preventable Standards Diseases Surveillance Standards
Mumps Mumps Vaccine Preventable Diseases 1 SurveillanceWHO Vaccine-Preventable Standards Diseases Surveillance Standards Mumps DISEASE AND VACCINE CHARACTERISTICS Mumps, caused by a paramyxovirus, is generally a mild and rubella (MMR), with two doses recommended. The disease with fever, headache and swelling of the salivary WHO position paper on mumps states the following: glands (parotitis); however, complications may occur “Routine mumps vaccination is recommended in such as meningitis, encephalitis and orchitis among countries with a well-established, effective childhood males, and mastitis and oophoritis among females. vaccination programme and the capacity to maintain Mumps is a leading cause of acquired sensorineural high-level vaccination coverage with measles and rubella deafness among children. Humans are the only known vaccination (that is, coverage that is > 80%) and where natural host for mumps virus, which is spread via the reduction of mumps incidence is a public health direct contact or by airborne droplets from the upper priority” (1). respiratory tract of infected individuals. The mumps vaccine is a live attenuated viral vaccine; several vaccine formulations are available. Mumps vaccine is most often given in combination with measles FIGURE Timing of mumps infectivity and specimen collection 1 PAROTITIS ONSET DAYS -25 ... -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Approximate exposure period Infectious period INFECTIVITY Previously vaccinated persons Unvaccinated persons COLLECTION BUCCAL SWAB SWAB BUCCAL Previously vaccinated M persons g I Unvaccinated SEROLOGY persons Solid colors indicate optimal time window. Gradient colors indicate when tests may provide accurate results. -

Immunization Recommendations for College Students
OCTOBER 2018 ACHA Guidelines Immunization Recommendations for College Students mmunizations offer safe and effective protection from vaccine-preventable diseases and outbreaks. The United States is experi- encing re-emergence of these diseases, in part due to factors such as un-immunized and under-immunized persons and global travel. I The American College Health Association (ACHA) strongly supports the use of vaccines to protect the health of our individual students and our campus communities. In recognition of the vital role that vaccine coverage plays in community immunity (herd immunity), ACHA discourages use of nonmedical exemptions to required vaccines. This guidance is provided to facilitate implementation of a comprehensive institutional immunization policy. Best practices for institu- tions of higher education include the following Immunization Recommendations for College Students (IRCS), encouraging students who request nonmedical exemptions to required vaccines to be counseled by a health service clinician, and considering exclusion of un- immunized students from school during outbreaks of vaccine-preventable diseases. Institutions may also be subject to additional requirements for pre-matriculation vaccinations and the granting of exemptions by state law. The ACHA Vaccine-Preventable Diseases Advisory Committee updates this document in accordance with changing public health rec- ommendations. These guidelines follow Advisory Committee on Immunization Practices (ACIP) recommendations published by the U.S. Centers for Disease -

Do Not Use to Submit Information To
University Health Services at UW-Madison Welcome, Test Patient | Logout ENTRANCE FORM: Immunizations Please see Frequently Asked Questions About Your Immunization and Health History Forms for more information. Please complete this form and provide the date(s) you received vaccines in childhood or as an adult. Not all students will have received all of these immunizations. If you do not enter any dates for an immunization, it will be For interpreted as your reporting that you have not received any doses of it. There are no required immunizations for enrollment in most programs at UW-Madison. However, University review Health Services strongly recommends the immunizations that follow to safeguard your health and the health of the campus. Certain health profession programs at UW-Madison do have immunization requirements, including hepatitis B, varicella (chickenpox), and tuberculosis. Please check with your program for exact requirements. purposesImportant note: If you will live in a university residence hall, you must complete the sections for hepatitis B and meningococcal vaccine prior to move-in. By Wisconsin state law, you must report whether or not you have received both vaccines. Failure to provide this information is a violation of your housing contract. We recommend that you print out this form for review prior to completing and submitting it. Once you have submitted the form, you cannot return to add or modify the information. Do not mail the form to us—only forms submitted through this website will be accepted. You may print out onlythis form and take it to your clinician to fill in the information for you. -

Chapter 15: Mumps; Epidemiology and Prevention of Vaccine
Mumps Mariel Marlow, PhD, MPH; Penina Haber, MPH; Carole Hickman, PhD; and Manisha Patel, MD, MS Mumps is an acute viral illness. Parotitis and orchitis were Mumps described by Hippocrates in the 5th century BCE. In 1934, ● Acute viral illness Claud Johnson and Ernest Goodpasture showed that mumps could be transmitted from infected patients to rhesus monkeys ● Parotitis and orchitis described by Hippocrates and demonstrated that mumps was caused by a filterable in 5th century BCE agent present in saliva. This agent was shown to be a virus in 1935. Mumps was one of the most common causes of aseptic ● Viral etiology described meningitis and sensorineural hearing loss in childhood in the by Johnson and Goodpasture United States until the introduction of a vaccine in 1967. In in 1934 1971, mumps vaccine was licensed in the United States as a ● Before vaccine, one of the combined measles, mumps, and rubella (MMR) vaccine. In 2005, most common causes of a combination measles, mumps, rubella, and varicella (MMRV) aseptic meningitis and hearing vaccine was licensed. loss among children and hospitalization among military During World War I, only influenza and gonorrhea were more ● Vaccination led to common than mumps as causes of hospitalization among over 99% reduction in soldiers. A successful 2-dose vaccination program in the United mumps cases States led to a greater than 99% reduction in the number of mumps cases reported annually. However, starting in 2006, there has been an increase in mumps cases and outbreaks, particularly in close-contact settings, with many occurring among fully vaccinated persons. Mumps Virus 15 Mumps Virus Mumps virus is a paramyxovirus in the same group as ● Paramyxovirus (RNA) parainfluenza and Newcastle disease viruses, which produce antibodies that cross-react with mumps virus. -

History of Immunization in BC
History of Immunization in BC School Immunization Programs Vaccine Year Group Rubella 1974-1985 Grade 5 girls (birth year cohort 1964-1975) Measles/Mumps/Rubella (MMR) 1986 Catch-up program for all children from Kindergarten to grade 12 Hepatitis B 1992-2012 (Jun) Grade 6 (birth year cohort 1981) Measles/Rubella (MR) 1996 Elementary through post-secondary school students (birth year cohort 1979-1991) Hepatitis B 1997 Grade 12 (birth year cohort 1980) Hepatitis B (2-dose schedule) 2001-2012 (Jun) Grade 6 (birth year cohort 1990) Hepatitis B (thimerosal-free) 2003-2012 (Jun) Grade 6 (birth year cohort 1992) Meningococcal C conjugate 2003-2016 (Jun) Grade 6 (birth year cohort 1992) Meningococcal C conjugate 2004-2006 (Jun) Grade 9 (birth year cohort 1990-1991) Tetanus, diphtheria, acellular 2004-present Grade 9 (replaced Td booster) pertussis (birth year cohort 1989) Varicella 2004-present Susceptible children at school entry and grade 6 (birth year cohorts 1999 and 1993) Meningococcal C conjugate 2005-2007 (Jun) Grade 12 (birth year cohort 1988-1989) Human papillomavirus (HPV) 2008-present Grade 6 girls (birth year cohort 1997) Human papillomavirus (HPV) 2008-2011 (Jun) Grade 9 girls (birth year cohort 1994) Program planned for 3 school years only (i.e., 2008/09, 2009/10 and 2010/11) Human papillomavirus (HPV) 2014 (Oct) 2-dose schedule for girls in grade 6 Meningococcal quadrivalent 2016 (Sep) Grade 9 (birth year cohort 2002) conjugate Human papillomavirus (HPV) 2016 (Sep) Gardasil®9 replaces Gardasil® for girls in grade 6 (birth year cohort 2005) Human papillomavirus (HPV) 2017 (Aug) Grade 6 boys (birth year cohort 2006) Indications/Comments in the following tables refer to new groups added to those for whom the vaccine is already indicated, unless otherwise stated.