Cnidae Variability in Balanophyllia Europaea and B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Deep‐Sea Coral Taxa in the U.S. Gulf of Mexico: Depth and Geographical Distribution
Deep‐Sea Coral Taxa in the U.S. Gulf of Mexico: Depth and Geographical Distribution by Peter J. Etnoyer1 and Stephen D. Cairns2 1. NOAA Center for Coastal Monitoring and Assessment, National Centers for Coastal Ocean Science, Charleston, SC 2. National Museum of Natural History, Smithsonian Institution, Washington, DC This annex to the U.S. Gulf of Mexico chapter in “The State of Deep‐Sea Coral Ecosystems of the United States” provides a list of deep‐sea coral taxa in the Phylum Cnidaria, Classes Anthozoa and Hydrozoa, known to occur in the waters of the Gulf of Mexico (Figure 1). Deep‐sea corals are defined as azooxanthellate, heterotrophic coral species occurring in waters 50 m deep or more. Details are provided on the vertical and geographic extent of each species (Table 1). This list is adapted from species lists presented in ʺBiodiversity of the Gulf of Mexicoʺ (Felder & Camp 2009), which inventoried species found throughout the entire Gulf of Mexico including areas outside U.S. waters. Taxonomic names are generally those currently accepted in the World Register of Marine Species (WoRMS), and are arranged by order, and alphabetically within order by suborder (if applicable), family, genus, and species. Data sources (references) listed are those principally used to establish geographic and depth distribution. Only those species found within the U.S. Gulf of Mexico Exclusive Economic Zone are presented here. Information from recent studies that have expanded the known range of species into the U.S. Gulf of Mexico have been included. The total number of species of deep‐sea corals documented for the U.S. -

Sexual Reproduction of the Solitary Sunset Cup Coral Leptopsammia Pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean
Marine Biology (2005) 147: 485–495 DOI 10.1007/s00227-005-1567-z RESEARCH ARTICLE S. Goffredo Æ J. Radetic´Æ V. Airi Æ F. Zaccanti Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis Received: 16 July 2004 / Accepted: 18 December 2004 / Published online: 3 March 2005 Ó Springer-Verlag 2005 Abstract Information on the reproduction in scleractin- came indented, assuming a sickle or dome shape. We can ian solitary corals and in those living in temperate zones hypothesize that the nucleus’ migration and change of is notably scant. Leptopsammia pruvoti is a solitary coral shape may have to do with facilitating fertilization and living in the Mediterranean Sea and along Atlantic determining the future embryonic axis. During oogene- coasts from Portugal to southern England. This coral sis, oocyte diameter increased from a minimum of 20 lm lives in shaded habitats, from the surface to 70 m in during the immature stage to a maximum of 680 lm depth, reaching population densities of >17,000 indi- when mature. Embryogenesis took place in the coelen- viduals mÀ2. In this paper, we discuss the morphological teron. We did not see any evidence that even hinted at aspects of sexual reproduction in this species. In a sep- the formation of a blastocoel; embryonic development arate paper, we report the quantitative data on the an- proceeded via stereoblastulae with superficial cleavage. nual reproductive cycle and make an interspecific Gastrulation took place by delamination. Early and late comparison of reproductive traits among Dend- embryos had diameters of 204–724 lm and 290–736 lm, rophylliidae aimed at defining different reproductive respectively. -

A Preliminary Study on Habitat Characteristics and Substrate Preference of Coral Species Distributed in the Dardanelles
Mar. Sci. Tech. Bull. (2014) 3(2):5-10 ISSN: 2147-9666 A preliminary study on habitat characteristics and substrate preference of coral species distributed in the Dardanelles. H. Baris Ozalp1*, Firat Sengun2, Zeki Karaca2, Olcay Hisar1 1Canakkale Onsekiz Mart University, Faculty of Marine Science and Technology, Canakkale, Turkey 2Canakkale Onsekiz Mart University, Faculty of Engineering, Canakkale, Turkey A R T I C L E I N F O A B S T R A C T Article history: The present study aims to investigate the characteristics of living substrata of two scleractinian corals in the Dardanelles by performing the ecologic and petrographical Received: 14.08.2014 analyses. The Mediterranean originated Caryophyllia smithii and Balanophyllia Received in revised form: 22.09.2014 europaea are frequent coral species in the Dardanelles and the studied area between Accepted : 07.10.2014 11 and 25 m depth is known as highly distributed zone of the species. In situ, the spreading area was searched by scuba technique and the ecological characteristics of Available online : 31.12.2014 species have been measured. For the purpose of petrographical analyses, six coral individuals were collected with the associated rock samples. According to the obtained Keywords: data it was determined that the rock samples are mainly composed of sandstone, micritic limestone and rhyolithic tuff. Additionally it was found that porosity in rock Coral structure has a positive effect on coral settlement and the habitat choice in B. Balanophyllia europaea europaea and C. smithii can differ based on the rock structure. Ecological, biological Caryophyllia smithii and zoogeographical features of Anthozoan species distributed in the Turkish coasts petrography are still poorly known. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Deep-Sea Coral Taxa in the U. S. Caribbean Region: Depth And
Deep‐Sea Coral Taxa in the U. S. Caribbean Region: Depth and Geographical Distribution By Stephen D. Cairns1 1. National Museum of Natural History, Smithsonian Institution, Washington, DC An update of the status of the azooxanthellate, heterotrophic coral species that occur predominantly deeper than 50 m in the U.S. Caribbean territories is not given in this volume because of lack of significant additional data. However, an updated list of deep‐sea coral species in Phylum Cnidaria, Classes Anthozoa and Hydrozoa, from the Caribbean region (Figure 1) is presented below. Details are provided on depth ranges and known geographic distributions within the region (Table 1). This list is adapted from Lutz & Ginsburg (2007, Appendix 8.1) in that it is restricted to the U. S. territories in the Caribbean, i.e., Puerto Rico, U. S. Virgin Islands, and Navassa Island, not the entire Caribbean and Bahamian region. Thus, this list is significantly shorter. The list has also been reordered alphabetically by family, rather than species, to be consistent with other regional lists in this volume, and authorship and publication dates have been added. Also, Antipathes americana is now properly assigned to the genus Stylopathes, and Stylaster profundus to the genus Stenohelia. Furthermore, many of the geographic ranges have been clarified and validated. Since 2007 there have been 20 species additions to the U.S. territories list (indicated with blue shading in the list), mostly due to unpublished specimens from NMNH collections. As a result of this update, there are now known to be: 12 species of Antipatharia, 45 species of Scleractinia, 47 species of Octocorallia (three with incomplete taxonomy), and 14 species of Stylasteridae, for a total of 118 species found in the relatively small geographic region of U. -

The Earliest Diverging Extant Scleractinian Corals Recovered by Mitochondrial Genomes Isabela G
www.nature.com/scientificreports OPEN The earliest diverging extant scleractinian corals recovered by mitochondrial genomes Isabela G. L. Seiblitz1,2*, Kátia C. C. Capel2, Jarosław Stolarski3, Zheng Bin Randolph Quek4, Danwei Huang4,5 & Marcelo V. Kitahara1,2 Evolutionary reconstructions of scleractinian corals have a discrepant proportion of zooxanthellate reef-building species in relation to their azooxanthellate deep-sea counterparts. In particular, the earliest diverging “Basal” lineage remains poorly studied compared to “Robust” and “Complex” corals. The lack of data from corals other than reef-building species impairs a broader understanding of scleractinian evolution. Here, based on complete mitogenomes, the early onset of azooxanthellate corals is explored focusing on one of the most morphologically distinct families, Micrabaciidae. Sequenced on both Illumina and Sanger platforms, mitogenomes of four micrabaciids range from 19,048 to 19,542 bp and have gene content and order similar to the majority of scleractinians. Phylogenies containing all mitochondrial genes confrm the monophyly of Micrabaciidae as a sister group to the rest of Scleractinia. This topology not only corroborates the hypothesis of a solitary and azooxanthellate ancestor for the order, but also agrees with the unique skeletal microstructure previously found in the family. Moreover, the early-diverging position of micrabaciids followed by gardineriids reinforces the previously observed macromorphological similarities between micrabaciids and Corallimorpharia as -

AC27 Doc. 12.5
Original language: English AC27 Doc. 12.5 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Interpretation and implementation of the Convention Review of Significant Trade in specimens of Appendix-II species [Resolution Conf. 12.8 (Rev. CoP13)] SELECTION OF SPECIES FOR TRADE REVIEWS FOLLOWING COP16 1. This document has been prepared by the Secretariat. 2. In Resolution Conf. 12.8 (Rev. CoP13) on Review of Significant Trade in specimens of Appendix-II species, the Conference of the Parties: DIRECTS the Animals and Plants Committees, in cooperation with the Secretariat and experts, and in consultation with range States, to review the biological, trade and other relevant information on Appendix-II species subject to significant levels of trade, to identify problems and solutions concerning the implementation of Article IV, paragraphs 2 (a), 3 and 6 (a)... 3. In accordance with paragraph a) of that Resolution under the section Regarding conduct of the Review of Significant Trade, the Secretariat requested UNEP-WCMC to produce a summary from the CITES Trade Database of annual report statistics showing the recorded net level of exports for Appendix-II species over the five most recent years. Its report is attached as Annex 1 (English only) to the present document. The raw data used to prepare this summary are available in document AC27 Inf. 2. 4. Paragraph b) of the same section directs the Animals Committee, on the basis of recorded trade levels and information available to it, the Secretariat, Parties or other relevant experts, to select species of priority concern for review (whether or not such species have been the subject of a previous review). -

Skeletal Mechanical Properties of Mediterranean Corals Along a Wide Latitudinal Gradient
Coral Reefs DOI 10.1007/s00338-014-1222-6 REPORT Skeletal mechanical properties of Mediterranean corals along a wide latitudinal gradient S. Goffredo • A. Mancuso • E. Caroselli • F. Prada • Z. Dubinsky • G. Falini • O. Levy • P. Fantazzini • L. Pasquini Received: 21 November 2013 / Accepted: 29 September 2014 Ó Springer-Verlag Berlin Heidelberg 2014 Abstract This is the first study exploring skeletal reduced skeletal bulk density and porosity with SST mechanical properties in temperate corals. The variation of (decreasing latitude) in B. europaea, and with the lack of skeletal mechanical properties with distance from the correlations with SST and latitude for skeletal bulk density aboral pole, coral age, and population was investigated in and porosity in L. pruvoti. These results support previous the corals Balanophyllia europaea (zooxanthellate) and hypotheses on differences in the skeletal mechanical Leptopsammia pruvoti (non-zooxanthellate), collected at properties of these two coral species in relation to the three sites along a wide latitudinal gradient. Mechanical observed variations of skeletal bulk density and porosity properties were measured by nanoindentation, a technique with temperature/latitude, which may have consequences applied here in detail for the first time to a scleractinian. In related to the envisaged seawater warming of next decades. both species, a reduction of Young’s modulus was observed toward the oral pole, which is the youngest part of Keywords Dendrophylliidae Á Elastic deformation Á the skeleton. Skeletons of B. europaea increased their Global warming Á Plastic deformation Á Scleractinia Á Young’s modulus with age, unlike L. pruvoti. Only the Skeletal resistance zooxanthellate species showed reduced Young’s modulus in southern populations, coherently with the observed Introduction Communicated by Handling Editor Brian Helmuth Sea surface temperature (SST) is one of the main factors Electronic supplementary material The online version of this that determine coral reef development (Kleypas et al. -
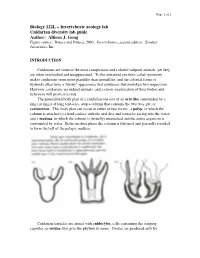
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

Spatial Distribution and the Effects of Competition on Some Temperate Scleractinia and Corallimorpharia
MARINE ECOLOGY PROGRESS SERIES Vol. 70: 39-48, 1991 Published February 14 Mar. Ecol. Prog. Ser. ~ Spatial distribution and the effects of competition on some temperate Scleractinia and Corallimorpharia Nanette E. Chadwick* Department of Zoology. University of California, Berkeley. California 94720. USA ABSTRACT: The impact of interference competition on coral community structure is poorly understood. On subtidal rocks in the northeastern Pacific, members of 3 scleractinian coral species (Astrangia lajollaensis, Balanophyllia elegans, Paracyathus stearnsii) and 1 corallimorpharian (Corynactls califor- nica) were examined to determine whether competition exerts substantial influence over the~rabund- ance and distributional patterns. These anthozoans occupy > 50 % cover on hard substrata, and exhibit characteristic patterns of spatial distribution, with vertical zonation and segregation among some species. They interact in an interspecific dominance hierarchy that lacks reversals, and is linear and consistent under laboratory and field conditions. Experiments demonstrated that a competitive domin- ant, C. californica, influences the abundance and population structure of a subordinate. B. elegans, by (1)reducing sexual reproductive output, (2) increasing larval mortality, (3)altering recruitment patterns. Field cross-transplants revealed that the dominant also affects vertical zonation of a competitive intermediate, A. lajollaensis, by lulling polyps that occur near the tops of subtidal rocks. It is concluded that between-species competition, -

Transcriptional Response of the Heat Shock Gene Hsp70 Aligns with Differences in Stress Susceptibility of Shallow-Water Corals from the T Mediterranean Sea
Marine Environmental Research 140 (2018) 444–454 Contents lists available at ScienceDirect Marine Environmental Research journal homepage: www.elsevier.com/locate/marenvrev Transcriptional response of the heat shock gene hsp70 aligns with differences in stress susceptibility of shallow-water corals from the T Mediterranean Sea ∗ Silvia Franzellittia, ,1, Valentina Airib,1, Diana Calbuccia, Erik Carosellib, Fiorella Pradab, Christian R. Voolstrac, Tali Massd, Giuseppe Falinie, Elena Fabbria, Stefano Goffredob a Animal and Environmental Physiology Laboratory, Department of Biological, Geological and Environmental Sciences, University of Bologna, via S. Alberto 163, I-48123, Ravenna, Italy b Marine Science Group, Department of Biological, Geological and Environmental Sciences, University of Bologna, Via F. Selmi 3, I-40126, Bologna, Italy c Red Sea Research Center, Division of Biological and Environmental Science and Engineering (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia d Department of Marine Biology, The Leon H. Charney School of Marine Sciences, University of Haifa, Multi Purpose Boulevard, Mt. Carmel, Haifa, 3498838, Israel e Department of Chemistry “Giacomo Ciamician”, University of Bologna, via F. Selmi 2, I-40126, Bologna, Italy ARTICLE INFO ABSTRACT Keywords: Shallow-water corals of the Mediterranean Sea are facing a dramatic increase in water temperature due to Thermal stress climate change, predicted to increase the frequency of bleaching and mass mortality events. However, suppo- Coral sedly not all corals are affected equally, as they show differences in stress susceptibility, as suggested by phy- Gene expression siological outputs of corals along temperature gradients and under controlled conditions in terms of reproduc- Heat shock protein tion, demography, growth, calcification, and photosynthetic efficiency.