And Bleph-10® (Sulfacetamide Sodium) Ophthalmic Ointment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brimonidine Tartrate; Brinzolamide
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate ; Brinzolamide This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate; Brinzolamide Dosage Form; Route: Suspension/drops; ophthalmic Strength: 0.2%; 1% Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm, in vivo Strength: 0.2%; 1% Subjects: Males and females with chronic open angle glaucoma or ocular hypertension in both eyes. Additional comments: Specific recommendations are provided below. ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open angle glaucoma and ocular hypertension comparing the test product to the reference listed drug (RLD), each applied as one drop in both eyes three times daily at approximately 8:00 a.m., 4:00 p.m., and 10:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open angle glaucoma or ocular hypertension in both eyes b. -

Allergy Testing and History Form
Skin Testing Information and Consent 1. Skin Testing An allergy skin test is used to identify the substances that are causing your allergy symptoms. We will apply several extracts of common allergens to the skin and observe for a reaction. The reactions are then graded and confirmatory intradermal testing may be performed. This involves placing a small amount of extract under the skin of the upper arm. We then observe the reaction and record the results. On the day of testing please wear a short-sleeved shirt that can be pushed up comfortably to your shoulder. Allow 1-2 hours for your test session. You will need to stay on the premises during this time. Please do not bring children to your appointment. 2. Risks of Skin Testing Bleeding and infection may occur due to abrading of the skin. Any time the skin integrity is broken it puts on at risk for infection. However, this is a rare occurrence. The antigens used for testing are sterile and approved by the FDA. Occasionally, skin testing can trigger a severe allergic reaction requiring treatment with medications and/or treatment in the ER. Patients with asthma are at increased risk for triggering an asthma attack during testing. You should not undergo testing if you feel that you have allergy or asthma symptoms are currently under poor control. 3. Contraindications to Skin Testing Women who are pregnant or anyone who is currently taking Beta-Blockers, Tricyclic Anti-depressants or MAOI’s medications will NOT be skin tested. Please be sure to inform us of ALL your medications before the skin test is applied. -

Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg
OPTOMETRIC STUDY CENTER Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg. % Product Sizes Side Effects Warnings Reduction CHOLINERGIC AGENTS Direct Pilocarpine (generic) Pilocarpine 1%, 2%, 4% Increases trabecular outflow BID-QID/15-25% 15ml Headache, blurred vision, myopia, retinal detachment, bronchiole constriction, Angle closure, shortness of breath, retinal narrowing of angle detachment Indirect Phospholine Iodide (Pfizer) Echothiophate iodide 0.125% Increases trabecular outflow QD-BID/15-25% 5ml Same as above plus cataractogenic iris cysts in children, pupillary block, Same as above, plus avoid prior to any increased paralysis with succinylcholine general anesthetic procedure ALPHA-2 AGONISTS Alphagan P (Allergan) Brimonidine tartrate 0.1%, 0.15% with Purite Decreases aqueous production, increases BID-TID/up to 26% 5ml, 10ml, 15ml Dry mouth, hypotension, bradycardia, follicular conjunctivitis, ocular irritation, Monitor for shortness of breath, dizziness, preservative uveoscleral outflow pruritus, dermatitis, conjunctival blanching, eyelid retraction, mydriasis, drug ocular redness and itching, fatigue allergy Brimonidine tartrate Brimonidine tartrate 0.15%, 0.2% Same as above Same as above 5ml, 10ml Same as above Same as above (generic) Iopidine (Novartis) Apraclonidine 0.5% Decreases aqueous production BID-TID/up to 25% 5ml, 10ml Same as above but higher drug allergy (40%) Same as above BETA-BLOCKERS Non-selective Betagan (Allergan) Levobunolol 0.25%, 0.5% Decreases -

Supporting Information a Analysed Substances
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 List of contents: Tab. A1 Detailed list and classification of analysed substances. Tab. A2 List of selected MS/MS parameters for the analytes. Tab. A1 Detailed list and classification of analysed substances. drug of therapeutic doping agent analytical standard substance abuse drug (WADA class)* supplier (+\-)-amphetamine ✓ ✓ S6 stimulants LGC (+\-)-methamphetamine ✓ S6 stimulants LGC (+\-)-3,4-methylenedioxymethamphetamine (MDMA) ✓ S6 stimulants LGC methylhexanamine (4-methylhexan-2-amine, DMAA) S6 stimulants Sigma cocaine ✓ ✓ S6 stimulants LGC methylphenidate ✓ ✓ S6 stimulants LGC nikethamide (N,N-diethylnicotinamide) ✓ S6 stimulants Aldrich strychnine S6 stimulants Sigma (-)-Δ9-tetrahydrocannabinol (THC) ✓ ✓ S8 cannabinoids LGC (-)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) S8 cannabinoids LGC morphine ✓ ✓ S7 narcotics LGC heroin (diacetylmorphine) ✓ ✓ S7 narcotics LGC hydrocodone ✓ ✓ Cerillant® oxycodone ✓ ✓ S7 narcotics LGC (+\-)-methadone ✓ ✓ S7 narcotics Cerillant® buprenorphine ✓ ✓ S7 narcotics Cerillant® fentanyl ✓ ✓ S7 narcotics LGC ketamine ✓ ✓ LGC phencyclidine (PCP) ✓ S0 non-approved substances LGC lysergic acid diethylamide (LSD) ✓ S0 non-approved substances LGC psilocybin ✓ S0 non-approved substances Cerillant® alprazolam ✓ ✓ LGC clonazepam ✓ ✓ Cerillant® flunitrazepam ✓ ✓ LGC zolpidem ✓ ✓ LGC VETRANAL™ boldenone (Δ1-testosterone / 1-dehydrotestosterone) ✓ S1 anabolic agents (Sigma-Aldrich) -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (Apraclonidine Hydrochloride) Eye Drops 0.5%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (apraclonidine hydrochloride) Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of Iopidine Eye Drops 0.5% contains apraclonidine hydrochloride 5.75 mg, equivalent to apraclonidine base 5 mg. Excipient with known effect Benzalkonium chloride 0.1 mg per 1 mL as a preservative. For the full list of excipients, see section 6.1. 2. PHARMACEUTICAL FORM Eye drops, solution, sterile, isotonic. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Iopidine Eye Drops 0.5% are indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Iopidine Eye Drops 0.5% to delay surgery should have frequent follow up examinations and treatment should be discontinued if the intraocular pressure rises significantly. The addition of Iopidine Eye Drops 0.5% to patients already using two aqueous suppressing drugs (i.e. beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because apraclonidine is an aqueous-suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP. The IOP-lowering efficacy of Iopidine Eye Drops 0.5% diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month. 4.2. Dose and method of administration Dose One drop of Iopidine Eye Drops 0.5% should be instilled into the affected eye(s) three times per day. -
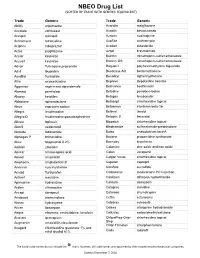
Drug List (SORTED by TRADE with GENERIC EQUIVALENT)
NBEO Drug List (SORTED BY TRADE WITH GENERIC EQUIVALENT) Trade Generic Trade Generic Abilify aripiprazole Avandia rosiglitazone Accolate zafirlukast Avastin bevacizumab Accupril quinapril Azasan azathioprine Achromycin tetracycline AzaSite azithromycin Aciphex rabeprazole Avodart dutasteride Actos pioglitazone Azopt brinzolamide Acular ketorolac Bactrim trimethoprim-sulfamethoxazole Acuvail ketorolac Bactrim DS trimethoprim-sulfamethoxazole Advair fluticasone propionate Baquacil polyhexamethylene biguanide Advil ibuprofen Beconase AQ beclomethasone AeroBid flunisolide Benadryl diphenhydramine Afrin oxymetazoline Bepreve Bepotastine besilate Aggrenox aspirin and dipyridamole Besivance besifloxacin Alamast pemirolast Betadine povidone-iodine Alaway ketotifen Betagan levobunolol Aldactone spironolactone Betasept chlorhexidine topical Aleve naproxen sodium Betaseron interferon beta-1b Allegra fexofenadine Betimol timolol Allegra-D fexofenadine-pseudoephedrine Betoptic S betaxolol Alluvia lopinavir Biopatch chlorhexidine topical Alocril nedocromil Blephamide sulfacetamide-prednisolone Alomide lodoxamide Botox onabotulinum toxinA Alphagan P brimonidine Brolene propamidine isethionate Alrex loteprednol 0.2% Bromday bromfenac Ambien zolpidem Calamine zinc oxide and iron oxide Amicar aminocaproic acid Calan verapamil Amoxil amoxicillin Calgon Vesta chlorhexidine topical Amphocin amphotericin B Capoten captopril Anectine succinylcholine Carafate sucralfate Ansaid flurbiprofen Carbocaine mepivacaine HCl injection Antivert meclizine Cardizem diltiazem -

IOPIDINE® 0.5% (Apraclonidine Ophthalmic Solution) 0.5% As Base
IOPIDINE® 0.5% (apraclonidine ophthalmic solution) 0.5% As Base DESCRIPTION: IOPIDINE® 0.5% Ophthalmic Solution contains apraclonidine hydrochloride, an alpha adrenergic agonist, in a sterile isotonic solution for topical application to the eye. Apraclonidine hydrochloride is a white to off-white powder and is highly soluble in water. Its chemical name is 2-[(4-amino-2,6 dichlorophenyl) imino]imidazolidine monohydrochloride with an empirical formula of C9H11Cl3N4 and a molecular weight of 281.57. The chemical structure of apraclonidine hydrochloride is: Each mL of IOPIDINE 0.5% Ophthalmic Solution contains: Active: apraclonidine hydrochloride 5.75 mg equivalent to apraclonidine base 5 mg; . Inactives: sodium chloride, sodium acetate, sodium hydroxide and/or hydrochloric acid (pH 4.4-7.8), purified water and benzalkonium chloride 0.01% (preservative) CLINICAL PHARMACOLOGY: Apraclonidine hydrochloride is a relatively selective alpha- 2-adrenergic agonist. When instilled in the eye, IOPIDINE 0.5% Ophthalmic Solution, has the action of reducing elevated, as well as normal, intraocular pressure (IOP), whether or not accompanied by glaucoma. Ophthalmic apraclonidine has minimal effect on cardiovascular parameters. Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. IOPIDINE 0.5% Ophthalmic Solution has the action of reducing IOP. The onset of action of apraclonidine can usually be noted within one hour, and maximum IOP reduction occurs about three hours after instillation. Aqueous fluorophotometry studies demonstrate that apraclonidine's predominant mechanism of action is reduction of aqueous flow via stimulation of the alpha-adrenergic system. -
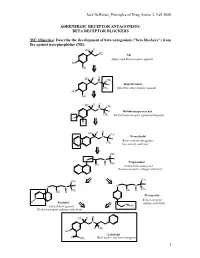
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

CARTEOLOL HYDROCHLORIDE- Carteolol Hydrochloride Solution Sandoz Inc ------Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile
CARTEOLOL HYDROCHLORIDE- carteolol hydrochloride solution Sandoz Inc ---------- Carteolol Hydrochloride Ophthalmic Solution USP, 1% Rx Only Sterile DESCRIPTION Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. The chemical name for carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2- hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows: C16H24N2O3•HCI Mol. Wt. 328.84 Each mL of sterile solution contains Active: carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water. CLINICAL PHARMACOLOGY Carteolol is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity. Carteolol Hydrochloride reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated. In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity. Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Carteolol Hydrochloride produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size. -

Glaucoma Medical Treatment: Philosophy, Principles and Practice
Glaucoma medical CLIVE MIGDAL treatment: philosophy, principles and practice Abstract assessment of these parameters. Indeed There have been numerous recent advances in compounds are under evaluation that affect the the management of glaucoma, not least the function of the optic nerve (via improved blood development of new drugs to help manage supply or improved neuronal cell physiology) raised intraocular pressure. In addition, the but may or may not lower lOP. It may even be concepts of improving blood flow to the optic possible in the future to therapeutically alter the nerve head and neuroprotection are currently human genome, genetically deliver provoking considerable interest. This article neuroprotective substances or aid regeneration considers the aims and philosophy of of the optic nerve axons. glaucoma drug therapy, summarises some of The main aim of glaucoma therapy must still the basic facts and principles of modem be the preservation of visual function. At the glaucoma medications, and suggests a same time, the therapy should not have adverse practical approach to the choice of therapy. side effects and should not affect the quality of life of the patient (by causing either side effects Key words Blood flow, Intraocular pressure, or inconvenience and disruption of daily Neuroprotection, Primary open angle glaucoma, Topical medications lifestyle). The cost of the therapy, both direct and indirect, must also be taken into consideration.s Currently, typical glaucoma management Philosophy consists of lowering the lOP to a satisfactory Primary open-angle glaucoma is a complex and safe target leve1.6 To determine the success disease for which a number of risk factors have of this treatment, the patient must be followed been identified, including intraocular pressure, long-term with routine assessment of lOP, discs age, race and family history.l,2 Due to our and fields to exclude progressive damage. -

REMEDY for GLAUCOMA COMPRISING Rho KINASE INHIBITOR and -BLOCKER
Europäisches Patentamt *EP001568382A1* (19) European Patent Office Office européen des brevets (11) EP 1 568 382 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: A61K 45/06, A61K 31/138, 31.08.2005 Bulletin 2005/35 A61K 31/343, A61K 31/353, A61K 31/437, A61K 31/4409, (21) Application number: 03772800.3 A61K 31/4412, A61K 31/496, (22) Date of filing: 17.11.2003 A61K 31/5377, A61K 31/551, A61P 27/06, A61P 43/00 (86) International application number: PCT/JP2003/014559 (87) International publication number: WO 2004/045644 (03.06.2004 Gazette 2004/23) (84) Designated Contracting States: • NAKAJIMA, Tadashi AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Ikoma-shi, Nara 630-0101 (JP) HU IE IT LI LU MC NL PT RO SE SI SK TR • MATSUGI, Takeshi Designated Extension States: Ikoma-shi, Nara 630-0101 (JP) AL LT LV MK • HARA, Hideaki Ikoma-shi, Nara 630-0101 (JP) (30) Priority: 18.11.2002 JP 2002333213 (74) Representative: Peaucelle, Chantal et al (71) Applicant: SANTEN PHARMACEUTICAL CO., Cabinet Armengaud Ainé LTD. 3, avenue Bugeaud Osaka 533-8651 (JP) 75116 Paris (FR) (72) Inventors: • HATANO, Masakazu Ikoma-shi, Nara 630-0101 (JP) (54) REMEDY FOR GLAUCOMA COMPRISING Rho KINASE INHIBITOR AND &bgr;-BLOCKER (57) A subject of the present invention is to find utility of a co mbination of a Rho kinase inhibitor having a novel action and a β-blocker as a therapeutic agent for glau- coma.