CTP Electronic Submission File Formats and Specifications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
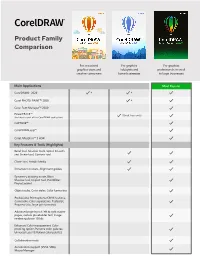
EXT EX Product Family Product Family for Occasional Graphics Users and Comparison Comparison Creative Consumers
TEXT EX Product Family Product Family For occasional graphics users and Comparison Comparison creative consumers For graphics hobbyists and home businesses For occasional For graphics For graphics graphics users and hobbyists and professionals in small Full-featured suite creative consumers home businesses to large businesses for graphics professionals in small to large businesses Main Applications Most Popular CorelDRAW® 2020 Corel PHOTO-PAINT™ 2020 Corel Font Manager™ 2020 PowerTRACE™ (Quick Trace only) (included as part of the CorelDRAW application) CAPTURE™ CorelDRAW.app™ Key Features & Tools (Highlights) Corel AfterShot™ 3 HDR Bevel tool, Shadow tools, Spiral, Smooth and Smear tool, Contour tool Key Features & Tools (Highlights) Clone Tool, Artistic Media Bevel tool, Shadow tools, Spiral, Smooth and Smear tool, Contour tool Key Features & Tools (Highlights) Dimension dockers, Alignment guides Clone Tool, Artistic Media Symmetry drawing mode, Block Shadow tool, Impact tool, Pointillizer, Dimension dockers, Alignment guides PhotoCocktail Barcode Wizard Symmetry drawing mode, Block Professional Print options Duplexing Wizard Shadow tool, Impact tool, Pointillizer, Remove (CMYK features, Composite, Color PhotoCocktail separations, Postscript, Prepress tabs) GPL Ghostscript for enhanced EPS Advanced page layout: left & right master Object styles, Color styles, Color harmonies and PS support pages, custom placeholder text, image rendering above 150dpi Professional Print options (CMYK features, Composite, Color separations, Postscript, Enhanced -

R-Photo User's Manual
User's Manual © R-Tools Technology Inc 2020. All rights reserved. www.r-tt.com © R-tools Technology Inc 2020. All rights reserved. No part of this User's Manual may be copied, altered, or transferred to, any other media without written, explicit consent from R-tools Technology Inc.. All brand or product names appearing herein are trademarks or registered trademarks of their respective holders. R-tools Technology Inc. has developed this User's Manual to the best of its knowledge, but does not guarantee that the program will fulfill all the desires of the user. No warranty is made in regard to specifications or features. R-tools Technology Inc. retains the right to make alterations to the content of this Manual without the obligation to inform third parties. Contents I Table of Contents I Start 1 II Quick Start Guide in 3 Steps 1 1 Step 1. Di.s..k.. .S..e..l.e..c..t.i.o..n.. .............................................................................................................. 1 2 Step 2. Fi.l.e..s.. .M..a..r..k.i.n..g.. ................................................................................................................ 4 3 Step 3. Re..c..o..v..e..r.y.. ...................................................................................................................... 6 III Features 9 1 File Sorti.n..g.. .............................................................................................................................. 9 2 File Sea.r.c..h.. ............................................................................................................................ -

Forcepoint DLP Supported File Formats and Size Limits
Forcepoint DLP Supported File Formats and Size Limits Supported File Formats and Size Limits | Forcepoint DLP | v8.8.1 This article provides a list of the file formats that can be analyzed by Forcepoint DLP, file formats from which content and meta data can be extracted, and the file size limits for network, endpoint, and discovery functions. See: ● Supported File Formats ● File Size Limits © 2021 Forcepoint LLC Supported File Formats Supported File Formats and Size Limits | Forcepoint DLP | v8.8.1 The following tables lists the file formats supported by Forcepoint DLP. File formats are in alphabetical order by format group. ● Archive For mats, page 3 ● Backup Formats, page 7 ● Business Intelligence (BI) and Analysis Formats, page 8 ● Computer-Aided Design Formats, page 9 ● Cryptography Formats, page 12 ● Database Formats, page 14 ● Desktop publishing formats, page 16 ● eBook/Audio book formats, page 17 ● Executable formats, page 18 ● Font formats, page 20 ● Graphics formats - general, page 21 ● Graphics formats - vector graphics, page 26 ● Library formats, page 29 ● Log formats, page 30 ● Mail formats, page 31 ● Multimedia formats, page 32 ● Object formats, page 37 ● Presentation formats, page 38 ● Project management formats, page 40 ● Spreadsheet formats, page 41 ● Text and markup formats, page 43 ● Word processing formats, page 45 ● Miscellaneous formats, page 53 Supported file formats are added and updated frequently. Key to support tables Symbol Description Y The format is supported N The format is not supported P Partial metadata -

Designing and Developing a Model for Converting Image Formats Using Java API for Comparative Study of Different Image Formats
International Journal of Scientific and Research Publications, Volume 4, Issue 7, July 2014 1 ISSN 2250-3153 Designing and developing a model for converting image formats using Java API for comparative study of different image formats Apurv Kantilal Pandya*, Dr. CK Kumbharana** * Research Scholar, Department of Computer Science, Saurashtra University, Rajkot. Gujarat, INDIA. Email: [email protected] ** Head, Department of Computer Science, Saurashtra University, Rajkot. Gujarat, INDIA. Email: [email protected] Abstract- Image is one of the most important techniques to Different requirement of compression in different area of image represent data very efficiently and effectively utilized since has produced various compression algorithms or image file ancient times. But to represent data in image format has number formats with time. These formats includes [2] ANI, ANIM, of problems. One of the major issues among all these problems is APNG, ART, BMP, BSAVE, CAL, CIN, CPC, CPT, DPX, size of image. The size of image varies from equipment to ECW, EXR, FITS, FLIC, FPX, GIF, HDRi, HEVC, ICER, equipment i.e. change in the camera and lens puts tremendous ICNS, ICO, ICS, ILBM, JBIG, JBIG2, JNG, JPEG, JPEG 2000, effect on the size of image. High speed growth in network and JPEG-LS, JPEG XR, MNG, MIFF, PAM, PCX, PGF, PICtor, communication technology has boosted the usage of image PNG, PSD, PSP, QTVR, RAS, BE, JPEG-HDR, Logluv TIFF, drastically and transfer of high quality image from one point to SGI, TGA, TIFF, WBMP, WebP, XBM, XCF, XPM, XWD. another point is the requirement of the time, hence image Above mentioned formats can be used to store different kind of compression has remained the consistent need of the domain. -
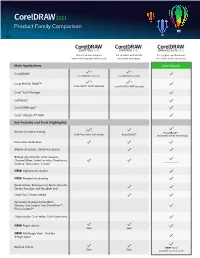
Coreldraw 2021 Product Family Comparison Matrix
TM Product Family Comparison For occasional graphics For graphics enthusiasts For graphics professionals users and those just starting out and home businesses in small to large businesses Main Applications Most Popular CorelDRAW® CorelDRAW Essentials CorelDRAW Standard Corel PHOTO-PAINT™ Corel PHOTO-PAINT Essentials Corel PHOTO-PAINT Standard Corel® Font Manager CAPTURE™ CorelDRAW.app™ Corel® AfterShot™ HDR Key Features and Tools (Highlights) ( ) Bitmap-to-vector tracing PowerTRACE™️ Quick Trace (one-click tracing) PowerTRACE™️ (Advanced with AI technology) Interactive Guidelines Alignment guides, Guidelines docker Bitmap adjustments: Color balance, Channel Mixer, Selective color, Desaturate, + many more! Gamma, Tone curve, + more NEW! Adjustments docker NEW! Perspective drawing Bevel docker, Shadow tool, Spiral, Smooth, Smear, Smudge, and Roughen tool Clone Tool, Artistic Media Symmetry drawing mode, Block Shadow tool, Impact tool, Pointillizer™, PhotoCocktail™ Object styles, Color styles, Color harmonies NEW! Pages docker Basic Basic NEW! Multipage View - Flexible design space Replace Colors NEW! More Basic Basic powerful, more intuitive Key Features and Tools (Highlights) Most Popular Enhanced Color management, Color proofing option, spot color support on output Professional Print options (CMYK features, Composite, Color separations, Postscript, Prepress tabs, large print preview) ENHANCED! Collaboration tools** Automation support (VSTA, VBA), Macro Manager Content Vector images 1,000 Over 7,000 High-resolution digital photos 100 1,000 -

27587 Apply Knowledge of Legislative Requirements in the Signmaking
NZQA registered unit standard 30160 version 1 Page 1 of 4 Title Demonstrate knowledge of and process vector and raster graphics files for sign production Level 4 Credits 30 Purpose This unit standard is for people working in the signmaking industry. People credited with this unit standard are able to demonstrate knowledge of graphic files and process vector and raster graphic files for sign production. Classification Sign Making > Sign Making - Core Available grade Achieved Explanatory notes 1 Reference Health and Safety at Work Act 2015. 2 File extension acronyms used: AI – Adobe illustrator file BMP – Bitmap CDL – Signlab vector graphics file CDR – Corel vector graphics file CPT – Corel photo paint file DXF – Drawing exchange file EPS – Encapsulated postscript vector graphics file FS – Flexisign file GIF – Graphics interchange format HPGL– HP graphics language plotter file JPEG– Joint photographic expert group PDF – Portable document format PNG – Portable network graphic PSD – Photoshop document RAW – Camera raw image file SVG – Scaleable vector graphics file TIFF – Tagged image file format 3 Definition Raster file – dot matrix pixel data structure representing an image. Workplace procedures – procedures used by the organisation carrying out the work and applicable to the tasks being carried out. Examples are – standard operating procedures, site safety procedures, equipment operating procedures, codes of Competenz New Zealand Qualifications Authority 2017 SSB Code 101571 NZQA registered unit standard 30160 version 1 Page 2 of 4 practice, quality management practices and standards, procedures to comply with legislative and local body requirements 4 Assessment information All evidence requirements must be performed in accordance with workplace procedures. Outcomes and evidence requirements Outcome 1 Demonstrate knowledge of graphic files for sign production. -

2020 Product Family Comparison
Product Family Comparison For occasional For graphics For graphics graphics users and hobbyists and professionals in small creative consumers home businesses to large businesses Main Applications Most Popular CorelDRAW® 2020 CorelDRAW Essentials 2020 CorelDRAW Standard 2020 Corel PHOTO-PAINT™ 2020 Corel PHOTO-PAINT Standard 2020 Corel Font Manager™ 2020 Remove PowerTRACE™️ CAPTURE™ CorelDRAW.app™ Corel AfterShot™ 3 HDR Key Features & Tools (Highlights) New row Bitmap-to-vector tracing ( ) Bitmap-to-vector tracing PowerTRACE™️ PowerTRACE™️ Quick Trace (one-click tracing) PowerTRACE™️ Bevel tool, Shadow tools, Spiral, Smooth Bevel tool, Shadow tool, Spiral, Smooth (Advanced with AI technology) (Advanced with AI technology) and Smear tool, Contour tool and Smear tool Bevel tool, Shadow tool, Spiral, Smooth and Smear tool Dimension dockers, Alignment guides Alignment guides, Guidelines docker Clone Tool, Artistic Media Alignment guides, Guidelines docker Symmetry drawing mode, Block Shadow tool, Impact tool, Pointillizer, PhotoCocktail Object styles, Color styles, Color harmonies Professional Print options (CMYK features, Composite, Color separations, Postscript, Prepress tabs, large print preview) Advanced page layout: left & right master pages, custom placeholder text, image Advanced page layout: left & right master rendering above 150dpi pages, custom placeholder text Enhanced Color management, Color Enhanced Color management, Color Enhanced Color management, Color proofing option, Pantone color palettes, proofing option, spot color support -

JPEG, TIFF, PSD, PDD, BMP, PICT, PNG, and GIF Explained
JPEG, TIFF, PSD, PDD, BMP, PICT, PNG, and GIF Explained I need to learn which format is best for saving my photos: JPEG, TIFF, PSD, PDD, BMP, PICT, PNG. I really don't understand which format is best for what. Is there a list that would tell when to use which one, and explain why? Also, when I am saving [JPEG], a box comes up asking if I want to save it in Baseline, Baseline Optimized, or Progressive. I have NO idea which of these is best! Answer: Here are some general guidelines: If the images are for the Web or online, use JPEG, PNG, or GIF. If the images are for print, use TIFF (but- here in com tech unless I tell you other wise use jpeg) If you want to keep a version that remains editable, choose your software's native file format. (PSD for Photoshop, PSP for Paint Shop Pro, CPT for Corel Photo-Paint, etc.) Here are brief descriptions of common graphics file formats, with links to follow for more information: JPEG - JPEG is best for photos when you need to keep the file size small and don't mind giving up some quality for a significant reduction in size(Generally here in com tech we can’t see the difference). JPEG is not suitable for images with text, large blocks of color, or simple shapes, because crisp lines will blur and colors can shift. Only JPEG offers the options of Baseline, Baseline Optimized, or Progressive. Baseline (Standard) - This JPEG format is recognized by all Web browsers. -

Corel Freedom Graphics License Lower Cost 3068-16.Cdr
The Corel Freedom Graphics License Solution Use the entire affordable Corel graphics solution, use just what make sense for your business, or use Corel applications in conjunction with other graphics applications to improve productivity and creativity. DESIGNER™ software Paint Shop Pro Photoshop 3rd-party software ® With a Corel Freedom Graphics Liciense, you get access to any or all seven Corel graphics applications and plug-ins, one year of Maintenance and upgrade protection, and one full set of license media. Corel Freedom Graphics License—Consider the Value Corel Freedom Graphics License Most Common File Seven Applications Included - One LOW Price Product Description Compare to Extenstion Support > > ® ® ® All-in-one vector illustration, Adobe Illustrator ($499, Upgrade $169) - cdr, ai, dxf, dwg, cgm, wmf, dsf, CorelDRAW Graphics Suite 12 svg, pdf, plus 90 more import and graphics design and layout Adobe Creative Suite ($999, Upgrade $549) export formats Quark XPress ($1,045, Upgrade $449) ® ™ > Corel DESIGNER Precision drawing suite for > AutoCAD LT ($899), Deneba™ Canvas™ ($399, DXF, DWG, CGM (v. 1, 3, 4ACGM, 4WebCGM, 4GREX), SVG, AI, CDR, Technical Suite 12 Technical illustration Upgrade $299) Adobe Illustrator or Creative Suite PDF, JPG, TIF, GIF, PSD, EPS, DSF, DRW, DST, MGX, PLT, DOC, XLF, PPT Additional applications included in the DRAW Graphics and DESIGNER Technical Suites: ® > ® ® ) Corel PHOTO-PAINT 12 Photo editing and bitmap effects > Adobe Photoshop ($649, Upgrade $169 - cpt, psd, ppf, gif, eps, jpg, png, tiff, riff, -

CGT 141/CPT 141 Lecture 5 Wk 3 Images Formats: JPG, GIF, Agif and PNG
CGT 141/CPT 141 Lecture 5 Wk 3 Images Formats: JPG, GIF, aGIF and PNG Graphic Interchange File Format (GIF, pronounced “jif”, like peanut butter) and Animated GIF • Two “flavors” o GIF 87a and 89a GIF 87a features 1. 8-bit (256 colors), called a palletized or indexed image 2. Permits interlacing o Writes the file in such a way that the file can be opened before all data is downloaded. o Looks like a camera focusing when image loads. o Gives the perception in the browser that the image loads faster; when in reality the file takes just as long, whether interlaced or not. o Interlacing and progression (or progressive as it relates to JPG) are conceptually similar (although the details are different). 3. Uses lossless compression – meaning an exact replica of the file is created when the file is uncompressed. o Compression rations at about 5:1 o Compression used is called Lempel-ZivWelch (LZW) o LZW is part of a family of compression algorithms called the LZ family, developed by Abraham Lempel and Jakob Ziv o The LZ family of compressors is used in a variety of ways, including ZIP, Arc, z, and tar files. o While working for Unisys, Terry Welch created a special type of compression for working with indexed raster images (LZW), specifically designed for use by CompuServe on their online service/ o UniSys, who had threatened to “suit anyone distributing GIF file”, patented LZW compression. Generally, an empty threat as it relates to the end user and web developer, but was one of the reasons for the emergence of the PNG format. -

Coreldraw® International Design Contest (The “Contest”) OFFICIAL RULES
CorelDRAW® International Design Contest (the “Contest”) OFFICIAL RULES BY PARTICIPATING IN THE CONTEST, ENTRANTS ACCEPT AND AGREE TO BE BOUND BY THE FOLLOWING RULES: 1. NO PURCHASE, PAYMENT OR DONATION IS NECESSARY. A purchase, payment or donation will not increase or improve your chances of winning. 2. ELIGIBILITY: Employees, representatives and agents of Corel Corporation, its subsidiaries and affiliates (“Corel”) and their immediate families (and those living in the same household) are ineligible to participate. The Contest is subject to all federal, local, provincial, state and governmental laws, rules and regulations. Entrants must be over the age of majority in the jurisdiction in which they reside. The Contest is void in the Canadian province of Quebec, the U.S. States of Rhode Island, Maine, Florida, and Puerto Rico and where prohibited by law, rule or regulation. 3. DURATION OF CONTEST: The Contest is open continuously from 0000h EST on February 3, 2009 to 2359h EST on May 31st, 2009 Corel’s computer is the official time keeping device for the receipt of entries for this Contest. 4. HOW TO ENTER: Contestants can download a free trial version of CorelDRAW® Graphics Suite from the Corel website or use their existing version of CorelDRAW® and/or Corel® PHOTO-PAINT™ to create a new design that fits into one of the following six (6) categories: (i) Advertising/Marketing Material (ii) Promotional Items/Awards/Personalization, (iii) Vehicle Wraps, (iv) Textile and Fashion Design, (v) General Illustration and Fine Art. The sixth category, (vi) Student/Academic, is reserved for Entrants that are currently registered in a Secondary or Post Secondary Academic Institution. -

R-Undelete User's Manual
User's Manual © R-Tools Technology Inc. 2020. All rights reserved. www.r-tt.com © R-tools Technology Inc 2020 All rights reserved. No part of this User's Manual may be copied, altered, or transferred to, any other media without written, explicit consent from R-tools Technology Inc.. All brand or product names appearing herein are trademarks or registered trademarks of their respective holders. R-tools Technology Inc. has developed this User's Manual to the best of its knowledge, but does not guarantee that the program will fulfill all the desires of the user. No warranty is made in regard to specifications or features. R-tools Technology Inc. retains the right to make alterations to the content of this Manual without the obligation to inform third parties. I R-Undelete Manual Table of Contents I Start 1 II Quick Start Guide in 3 Steps 1 1 Step 1. Di.s..k. .S...e..l.e..c.t.i.o..n.. ............................................................................................................... 1 2 Step 2. Fi.l.e.. .M...a..r.k..i.n..g.. ................................................................................................................. 4 3 Step 3. Re..c..o..v..e..r.y.. ...................................................................................................................... 5 III Features 9 1 File Sortin..g.. ............................................................................................................................... 9 2 File Sea.r.c..h.. ............................................................................................................................