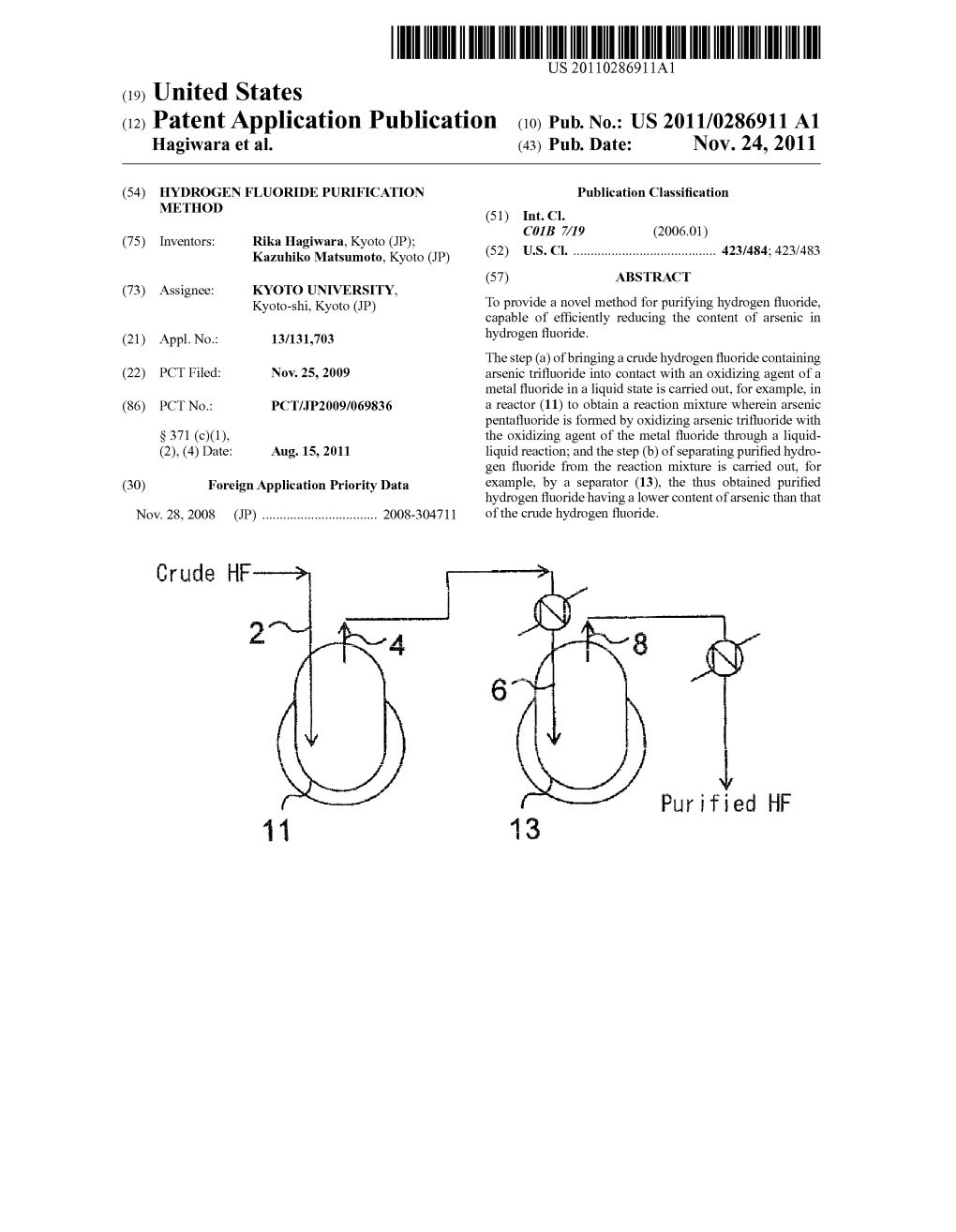
Crude Lf -> - 2^- 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Safety Manual
LABORATORY SAFETY MANUAL Environment, Health & Safety 1120 Estes Drive Extension CB# 1650 TABLE OF CONTENTS Section or Chapter Page Introduction i Emergency Telephone Numbers ii EHS – Scope of Service iii Condensed Laboratory Safety Information for New Research Personnel v Chapter 1 – Laboratory Safety at the University of North Carolina at Chapel Hill 1-1 Chapter 2 – Laboratory Safety Plan 2-1 Chapter 3 – General Safety Principles and Practices 3-1 Chapter 4 – Proper Storage of Chemicals in Laboratories 4-1 Chapter 5 – Protective Clothing and Equipment 5-1 Chapter 6 – Safe Handling of Chemicals 6-1 Chapter 7 – Highly Toxic Chemicals and Select Carcinogens 7-1 Chapter 8 – Reproductive Health 8-1 Chapter 9 – Controlled Substances 9-1 Chapter 10 – Fire Safety 10-1 Chapter 11 – Explosive and Reactive Chemical Hazards 11-1 Chapter 12 – Management of Laboratory Wastes 12-1 Chapter 13 – Safe Handling of Peroxidizable Compounds 13-1 Chapter 14 – Safe Handling of Laboratory Animals 14-1 Chapter 15 – Safe Handling of Biological Hazards 15-1 Chapter 16 – Biological Safety Cabinets 16-1 Chapter 17 – Laboratory Hoods 17-1 Chapter 18 – Safe Use of Nanomaterials 18-1 Revisions to Laboratory Safety Manual REV-1 Laboratory Safety Manual – the University of North Carolina at Chapel Hill INTRODUCTION This manual is a safety reference document for laboratory personnel at the University of North Carolina at Chapel Hill. The University’s Department of Environment, Health & Safety prepared this manual, followed by review and approval from both the University’s Laboratory and Chemical Safety Committee (LCSC) and the University Safety and Security Committee (USSC). -

Benzene MSDS
He a lt h 2 3 Fire 3 2 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n H Material Safety Data Sheet Benzene MSDS Section 1: Chemical Product and Company Identification Product Name: Benzene Contact Information: Catalog Codes: SLB1564, SLB3055, SLB2881 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: 71-43-2 Houston, Texas 77396 RTECS: CY1400000 US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Benzene Order Online: ScienceLab.com CI#: Not available. CHEMTREC (24HR Emergency Telephone), call: Synonym: Benzol; Benzine 1-800-424-9300 Chemical Name: Benzene International CHEMTREC, call: 1-703-527-3887 Chemical Formula: C6-H6 For non-emergency assistance, call: 1-281-441-4400 Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Benzene 71-43-2 100 Toxicological Data on Ingredients: Benzene: ORAL (LD50): Acute: 930 mg/kg [Rat]. 4700 mg/kg [Mouse]. DERMAL (LD50): Acute: >9400 mg/kg [Rabbit]. VAPOR (LC50): Acute: 10000 ppm 7 hours [Rat]. Section 3: Hazards Identification Potential Acute Health Effects: Very hazardous in case of eye contact (irritant), of inhalation. Hazardous in case of skin contact (irritant, permeator), of ingestion. Inflammation of the eye is characterized by redness, watering, and itching. Potential Chronic Health Effects: CARCINOGENIC EFFECTS: Classified A1 (Confirmed for human.) by ACGIH, 1 (Proven for human.) by IARC. MUTAGENIC EFFECTS: Classified POSSIBLE for human. Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female [POSSIBLE]. -

Arsenic Pentafluoride Sds- Material Safety Data Sheet
Tel: 514-956-7503 Fax: 514-956-7504 Internet: www.megs.ca E-mail: [email protected] Montreal St-Laurent Tel : 514-956-7503 Fax : 514-956-7504 Ottawa Nepean Tel : 613-226-4228 Fax : 613-226-4229 Quebec Quebec Tel : 418-834-7447 Fax : 418-834-3774 ARSENIC PENTAFLUORIDE SDS- MATERIAL SAFETY DATA SHEET TABLE OF CONTENTS: 1. Chemical Product and Company Identification 24 Hour EMERGENCY 2. Composition, Information on Ingredients CONTACT 3. Hazards Identification 4. First Aid Measures 5. Fire Fighting Measures U.S- CHEMTREC 1-800- 6. Accidental Release Measures 424-9300 7. Handling and Storage 8. Exposure Controls, Personal Protection CANADA- CANUTEC 613- 9. Physical and Chemical Properties 996-6666 10. Stability and Reactivity 11. Toxicological Information 12. Ecological Information 13. Disposal Considerations 14. Transport Information 15. Regulatory Information 16. Other Information 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION-----------Up to Table of Contents Matheson Tri-Gas, Inc. The telephone numbers listed below are emergency numbers, please contact your local branch for routine inquiries. USA CANADA 959 Route 46 East 530 Watson Street Parsippany, New Jersey Whitby, Ontario 07054-0624 USA L1N 5R9 Canada Phone: 973-257-1100 Phone: 905-668-3570 SUBSTANCE: ARSENIC PENTAFLUORIDE SDS SYMBOL: AsF5 TRADE NAMES/SYNONYMS: ARSORANE, PENTAFLUORO-; ARSENIC FLUORIDE (ASF5); ARSENIC FLUORIDE; ASF5; MAT02015 CHEMICAL FAMILY: non-metallic, halides CREATION DATE: Jan 24 1989 REVISION DATE: Mar 16 1999 2. COMPOSITION, INFORMATION ON INGREDIENTS-----------Up -

Elimination Des Gaz
ELIMINATION DES GAZ IGC Doc 30/13/E Révision du Doc 30/10/E EUROPEAN INDUSTRIAL GASES ASSOCIATION AISBL AVENUE DES ARTS 3-5 • B-1210 BRUSSELS Tel : +32 2 217 70 98 • Fax: +32 2 219 85 14 E-mail : [email protected] • Internet : http://www.eiga.eu ELIMINATION DES GAZ RÉVISÉ PAR : Jean-Paul Barbier AIR LIQUIDE Giorgio Bissolotti SIAD Kevin Cleaver THE LINDE GROUP Esteban Elias AIR PRODUCTS Joachim Barbe MESSER GROUP Pierre Wolfs EIGA Déclaration Toutes les publications techniques éditées par EIGA ou sous son égide, et notamment ses codes de bonne pratique, les guides de procédures en matière de sécurité et toutes autres informations techniques contenues dans ces publications ont été élaborées avec le plus grand soin et établies avec les connaissances acquises des membres de EIGA ou de tiers à la date de leur publication. Elles n’ont la valeur juridique que de simples recommandations que les membres de EIGA ou les tiers ne sont pas tenus contractuellement de respecter: Elles ne peuvent faire l’objet vis-à-vis de quiconque, d’aucune garantie de la part d’EIGA. EIGA n’a ni le pouvoir, ni les moyens de vérifier que les codes de bonne pratique et les guides de procédures sont effectivement et correctement interprétés et appliqués par l’utilisateur qui engage seul sa responsabilité à cet égard. En conséquence, EIGA ne saurait en aucun cas être tenu pour responsable vis-à-vis de quiconque, de l’application par ses membres ou par toute autre personne, de ses codes de bonne pratique et guides de procédure. -

A Review of Arsenic in Ambient Air in the UK
Report A Review of Arsenic in Ambient Air in the UK Prepared on behalf of : Department of the Environment, Transport and the Regions Scottish Executive The National Assembly for Wales February 2000 i Report A Review of Arsenic in Ambient Air in the UK Prepared By Richard Maggs - Principal Consultant Approved By Steve Moorcroft - Director Prepared for Department of the Environment, Transport and the Regions Scottish Executive The National Assembly for Wales February 2000 Our ref 305109 Your ref Document ref SSE/AQ/1465 ii Contents page 1. Introduction 7 2. Physical and Chemical Nature of Arsenic 7 2.1. Properties of Arsenic 7 2.2. Occurrence of Arsenic 8 3. The Atmospheric Chemistry of Arsenic 9 3.1. Particulate Arsenic 9 3.2. Vapour-phase Arsenic 10 4. UK Sources of Arsenic 12 4.1. Combustion Sources 12 4.2. Production Processes 13 4.2.1. Non-ferrous Metal Industry 14 4.2.2. Iron & Steel Industry 14 4.2.3. Sinter Plants 15 4.2.4. Non-ferrous Metal Mining 15 4.2.5. Waste Treatment and Disposal 15 4.2.6. Timber Industry 16 4.3. Emission Inventory Estimates 16 5. Air Quality Guidelines 20 6. Measurement Techniques 21 6.1. Sampling 21 6.2. Filter Media 21 6.3. Sample Preparation and analysis 22 6.3.1. Sample Preparation 23 6.3.2. Commonly Used Analytical Methods 24 6.4. Vapour-Phase Arsenic 25 6.5. Proposed Sampling Method 26 7. Monitoring in the UK 27 7.1. Rural Network Data 27 7.2. Urban Network Data 29 iii 7.3. -

Guidelines for the Preparation of Safety Data Sheets
GUIDELINES FOR THE PREPARATION OF SAFETY DATA SHEETS EIGA 919/14 Revision of EIGA 919/13 EUROPEAN INDUSTRIAL GASES ASSOCIATION AISBL AVENUE DES ARTS 3-5 • B – 1210 BRUSSELS Tel: +32 2 217 70 98 • Fax: +32 2 219 85 14 E-mail: [email protected] • Internet: www.eiga.eu EIGA 919/14 GUIDELINES FOR THE PREPARATION OF SAFETY DATA SHEETS PREPARED BY : Joachim Barbe Messer Group Ewoud Beheydt Praxair Gianluigi Colombo Sapio Produzione Idrogeno Ossigeno Joachim Eiselt Air Liquide Natalie Kingswood The Linde Group Zbigniew Sokolowski Air Products Ben Valk The Linde Group Pierre Wolfs EIGA Disclaimer All technical publications of EIGA or under EIGA's name, including Codes of practice, Safety procedures and any other technical information contained in such publications were obtained from sources believed to be reliable and are based on technical information and experience currently available from members of EIGA and others at the date of their issuance. While EIGA recommends reference to or use of its publications by its members, such reference to or use of EIGA's publications by its members or third parties are purely voluntary and not binding. Therefore, EIGA or its members make no guarantee of the results and assume no liability or responsibility in connection with the reference to or use of information or suggestions contained in EIGA's publications. EIGA has no control whatsoever as regards, performance or non performance, misinterpretation, proper or improper use of any information or suggestions contained in EIGA's publications by any person or entity (including EIGA members) and EIGA expressly disclaims any liability in connection thereto. -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Appendix B Classification of common chemicals by chemical band 1 1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3A1, R2A1C1, RA1C12 6 Examples: Et3A1, Et2A1C1, EtA.1111C12, Me3A1, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R 7 alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutylthhium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithiurn, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/A1203 26 1.13. Finely Divided Sulfides: -

By T. E. THORPE, F.R.S., and J. W. RODGER
View Article Online / Journal Homepage / Table of Contents for this issue 306 XXX1V.- On Thiophosp hory 1 Fluoride. By T. E. THORPE,F.R.S., and J. W. RODGER,Associate of the Normal School of Science, S. Kensington. INa short paper bearing the above title, published in the Transac- tions of the Society during the summer vacation of last yeir (Trans., 1888, 53, 766), we announced the existence OF a new gaseous sub- stance of the formula PSE',, to which we gave the name of thio- phosphory ZJluoride. The present communication contains the results of the experiments which have served to establish the nature and composition of the new compound. Arsenic trifluoride acts with great violence on phosphorus penta- chloride, wit'h the formation of phosphorus pentafluoride and arsenic trichloride (Thorpe, Proc. Boy. Xoc., 25,122). Phosphorus trichloride mixes with arsenic trifluoride, and on gently warming, the mixture evolves the gaseous phosphorus trifluoride (Moissan, Ann. Chim. Phys. [S], 6,433). The behaviour of thiophosphoryl chloride with arsenic trifluoride is however very different. If amenic trifluoride is dropped into phos- phorus thiochloride in the cold, or if a mixture of the two compouiids is boiled under ordinary pressures, no action takes place. When, however, the mixture is heated in a sealed glass tube at 150", arsenious sulphide separates, and on cooling and opening the tc;be considerable quantities of gas are evolved. The nature of tthe gaseous product varies with the relative proportions of the arsenic fluoride and phosphorus thiochloride. If the reacting substances are heated in the proportions demanded by the equation- Published on 01 January 1889. -

Nitrogen Tribromide Polar Or Nonpolar
Nitrogen tribromide polar or nonpolar Continue Nitrogen Tribromid Names IUPAC Name Nitrogen Tribromid Identifiers CAS Number 15162-90-0 3D Model (JSmol) Interactive Image ChemSpider 20480821 PubChem CID 3082084 CompTox Dashboard (EPA) DTXSID901648 In22 InChI InChIBrH.N/h3'1H;/p-3 SMILES N(Br)(Br)Br Properties Chemical Formula NBr3 Molar Mass 253.7187 g/mol Appearance Deep-red solid melting point explodes at -100 degrees Celsius, except when otherwise noted, the data is given for materials in their standard state (at 25 degrees Celsius), 100 kPa). Infobox links nitrogen tribromid is a chemical compound with the NBr3 formula. It is extremely explosive in its purest form, even at 100 degrees Celsius, and was not isolated until 1975. It's deep-red and volatile solid. The drug NBr3 was first prepared by the reaction of bistrimetligrilbramamin (bis (trimethylsil)amin bromide) with bromine monochloride (with trimethylylyl chloride as a by-product) at 87 degrees on the following equations: (Me3Si)2NBr2 BrCl → NBr3 and 2 Me3SiCl, Where Me is He instantly reacts with ammonia in a dichloromethane solution at 87 degrees Celsius to give NBrH2. Links - Lide, David R. (1998), Handbook on Chemistry and Physics (87 Ed.), Boca-Raton, Florida: CRC Press, p. 4-73, ISBN 0-8493-0594-2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements (2nd st. Butterworth-Keinmann. page 439. ISBN 978-0-08-037941-8. vteSalts and covalent derivatives of nitrid ion NH3N2H4 He (N2)11 Li3N Be3N2 BN β-C3N4g- C3N4CxNy N2 NxOy NF3 Ne Na3N Mg3 NN2 AlN Si3N4 PNP3N5 SxNySNS4N4 NCl3 Ar K3N Ca3N2 ScN VN CrNCr2N MnxNy FexNy Ni3N CuN n3N2 GaN Ge3N4 as Se NBr3 Kr Rb3 Yn Sr3N2 yn srn NbN β-Mo2N Tc Ru Rh PdN AgN CdN InN Sb Te NI3 Xe Cs3N Ba3N2 Hf3N4 TaN WN Re Os Au Hg3N2 TlN Pb BiN Po At At Rn Fr3N Ra3N2 Rf Db Sg Bh Hs Mt D rg Cn Nh Fl Mc Lv Ts Og s La CeN Pr Nd Pm Sm Eu GdN Tb Dy Er Tm Yb Lu Ac Th Pa UN Np Pu Am Cm Bk Cf Es Fm No Lr Lr Extracted from the Is NBr3 (Nitrogen Tribromid) polar or non-polar? NBr3 (Nitrogen Tribromid) is a polar I'll tell you the polar or nonpolar list below. -

Preparation and Properties of Tetrachlorophosphonium
This dissertation has been microfilmed exactly as received 66-1851 WALTHER, James Fletcher, 1938- PREPARATION AND PROPERTIES OF T E TRAC HLOROPHOSPHONIUM TETRACHLORODIFLUOROPHOSPHATE. The Ohio State University, Ph.D., 1965 Chemistry, inorganic University Microfilms, Inc., Ann Arbor, Michigan PREPARATION AND PROPERTIES OP TETRACHLOROPHOSPHONIUH TKTRACHLORGDIFLUQROPHOSPHATE DISSERTATION Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy In the Graduate School of The Ohio State University BSP James Fletcher Walther, B.A.* M.Sc* The Ohio State University 1965 Department of Chemistry ACKHCMI2DGMENTS I wish to express ngr sincerest gratitude to my adviser* Or. Sheldon Q. Shore* Tor suggesting this very interesting and perplexing research problem. I am dearly grateful to ay wife* Kay* for her faithful assistance in preparing this dissertation and for her enduring interest and enthusiasm in my work. Also* I want to express my appreciation to the Chemistry Department of The Ohio State University for its financial assist ance through various teaching assistantships and summer fellow ships. ii VITA August *** 1 9 3 8 ........... Born* St* Louis* Missouri i960 ••••••••• ••• B*A** Central Methodist College* Fayette* Missouri 1960-1963 ....................... Teaching Assistant* Department of Chemistry* The Ohio State University* Columbus* Ohio 1963-196**................... Teaching Assistant* Graduate level* Inorganic Division* Department of Chemistry The Ohio State University* Columbus* (Alio 196*4—1965 ..............