Characterizing the Waters of 6 Rivers in Upstate New York with a Focus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mohawk River Watershed – HUC-12
ID Number Name of Mohawk Watershed 1 Switz Kill 2 Flat Creek 3 Headwaters West Creek 4 Kayaderosseras Creek 5 Little Schoharie Creek 6 Headwaters Mohawk River 7 Headwaters Cayadutta Creek 8 Lansing Kill 9 North Creek 10 Little West Kill 11 Irish Creek 12 Auries Creek 13 Panther Creek 14 Hinckley Reservoir 15 Nowadaga Creek 16 Wheelers Creek 17 Middle Canajoharie Creek 18 Honnedaga 19 Roberts Creek 20 Headwaters Otsquago Creek 21 Mill Creek 22 Lewis Creek 23 Upper East Canada Creek 24 Shakers Creek 25 King Creek 26 Crane Creek 27 South Chuctanunda Creek 28 Middle Sprite Creek 29 Crum Creek 30 Upper Canajoharie Creek 31 Manor Kill 32 Vly Brook 33 West Kill 34 Headwaters Batavia Kill 35 Headwaters Flat Creek 36 Sterling Creek 37 Lower Ninemile Creek 38 Moyer Creek 39 Sixmile Creek 40 Cincinnati Creek 41 Reall Creek 42 Fourmile Brook 43 Poentic Kill 44 Wilsey Creek 45 Lower East Canada Creek 46 Middle Ninemile Creek 47 Gooseberry Creek 48 Mother Creek 49 Mud Creek 50 North Chuctanunda Creek 51 Wharton Hollow Creek 52 Wells Creek 53 Sandsea Kill 54 Middle East Canada Creek 55 Beaver Brook 56 Ferguson Creek 57 West Creek 58 Fort Plain 59 Ox Kill 60 Huntersfield Creek 61 Platter Kill 62 Headwaters Oriskany Creek 63 West Kill 64 Headwaters South Branch West Canada Creek 65 Fly Creek 66 Headwaters Alplaus Kill 67 Punch Kill 68 Schenevus Creek 69 Deans Creek 70 Evas Kill 71 Cripplebush Creek 72 Zimmerman Creek 73 Big Brook 74 North Creek 75 Upper Ninemile Creek 76 Yatesville Creek 77 Concklin Brook 78 Peck Lake-Caroga Creek 79 Metcalf Brook 80 Indian -

NYS Disaster Preparedness Commission
New York State Disaster Preparedness Commission 2013 Annual Report Prepared by the NYS Division of Homeland Security & Emergency Services Office of Emergency Management March 31, 2014 Andrew M. Cuomo Governor Jerome M. Hauer Chairman TABLE OF CONTENTS INTRODUCTION ............................................................................................................................................. 1 OVERVIEW ..................................................................................................................................................... 2 HIGHLIGHTS OF ACTIVITIES ........................................................................................................................... 3 February 8–9: Winter Storm “Nemo” ....................................................................................................... 3 June-July: Severe Weather / Repetitive Storms ....................................................................................... 6 PROGRAM STATUS ...................................................................................................................................... 12 Grant Administration .............................................................................................................................. 12 Operations .............................................................................................................................................. 13 Incident Management Team Program ................................................................................................... -

Freshwater Fishing: a Driver for Ecotourism
New York FRESHWATER April 2019 FISHINGDigest Fishing: A Sport For Everyone NY Fishing 101 page 10 A Female's Guide to Fishing page 30 A summary of 2019–2020 regulations and useful information for New York anglers www.dec.ny.gov Message from the Governor Freshwater Fishing: A Driver for Ecotourism New York State is committed to increasing and supporting a wide array of ecotourism initiatives, including freshwater fishing. Our approach is simple—we are strengthening our commitment to protect New York State’s vast natural resources while seeking compelling ways for people to enjoy the great outdoors in a socially and environmentally responsible manner. The result is sustainable economic activity based on a sincere appreciation of our state’s natural resources and the values they provide. We invite New Yorkers and visitors alike to enjoy our high-quality water resources. New York is blessed with fisheries resources across the state. Every day, we manage and protect these fisheries with an eye to the future. To date, New York has made substantial investments in our fishing access sites to ensure that boaters and anglers have safe and well-maintained parking areas, access points, and boat launch sites. In addition, we are currently investing an additional $3.2 million in waterway access in 2019, including: • New or renovated boat launch sites on Cayuga, Oneida, and Otisco lakes • Upgrades to existing launch sites on Cranberry Lake, Delaware River, Lake Placid, Lake Champlain, Lake Ontario, Chautauqua Lake and Fourth Lake. New York continues to improve and modernize our fish hatcheries. As Governor, I have committed $17 million to hatchery improvements. -
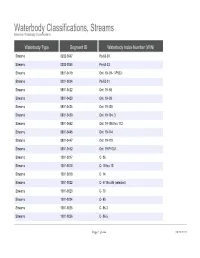
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

New York Fishing Regulations Guide: 2017-18
NEW YORK Freshwater FISHING 2017–18 O cial Volume 9, Issue No. 1 April 2017 Regulations Guide Fishing Lake Champlain www.dec.ny.gov Most regulations are in eect April 1, 2017 through March 31, 2018 Message from the Governor New York: A State of Angling Opportunity If you are an angler, you know how lucky you are to live in New York. The Empire State is unmatched in the quality and diversity of our fish species, and ranks fifth in the nation for total acreage of freshwater fishing locations. There are so many opportunities to get out and enjoy this important sport, and I have made it a priority to ensure that all New Yorkers have access to the State’s abundant fishing resources. That is why the NY is Open for Fishing and Hunting initiative has provided more than $4 million over the last three years to rehabilitate existing boat launches and construct new launches across the state. In addition, New York continues to make great progress toward our goal of establishing 50 new or improved land and water access sites. In 2016, we opened new universally accessible fishing piers and hand carry launches on the Esopus Creek in Ulster County and Looking Glass Pond in Schoharie County. We refurbished the Dunkirk Harbor Fishing Pier in Chautauqua County, which now provides access for users of all abilities to the outstanding fishing found in this area of Lake Erie. 2017 will see the opening of a brand new boat launch on Meacham Lake in Franklin County and updated facility on the northern end of Cayuga Lake. -

Distribution of Ddt, Chlordane, and Total Pcb's in Bed Sediments in the Hudson River Basin
NYES&E, Vol. 3, No. 1, Spring 1997 DISTRIBUTION OF DDT, CHLORDANE, AND TOTAL PCB'S IN BED SEDIMENTS IN THE HUDSON RIVER BASIN Patrick J. Phillips1, Karen Riva-Murray1, Hannah M. Hollister2, and Elizabeth A. Flanary1. 1U.S. Geological Survey, 425 Jordan Road, Troy NY 12180. 2Rensselaer Polytechnic Institute, Department of Earth and Environmental Sciences, Troy NY 12180. Abstract Data from streambed-sediment samples collected from 45 sites in the Hudson River Basin and analyzed for organochlorine compounds indicate that residues of DDT, chlordane, and PCB's can be detected even though use of these compounds has been banned for 10 or more years. Previous studies indicate that DDT and chlordane were widely used in a variety of land use settings in the basin, whereas PCB's were introduced into Hudson and Mohawk Rivers mostly as point discharges at a few locations. Detection limits for DDT and chlordane residues in this study were generally 1 µg/kg, and that for total PCB's was 50 µg/kg. Some form of DDT was detected in more than 60 percent of the samples, and some form of chlordane was found in about 30 percent; PCB's were found in about 33 percent of the samples. Median concentrations for p,p’- DDE (the DDT residue with the highest concentration) were highest in samples from sites representing urban areas (median concentration 5.3 µg/kg) and lower in samples from sites in large watersheds (1.25 µg/kg) and at sites in nonurban watersheds. (Urban watershed were defined as those with a population density of more than 60/km2; nonurban watersheds as those with a population density of less than 60/km2, and large watersheds as those encompassing more than 1,300 km2. -

Before Albany
Before Albany THE UNIVERSITY OF THE STATE OF NEW YORK Regents of the University ROBERT M. BENNETT, Chancellor, B.A., M.S. ...................................................... Tonawanda MERRYL H. TISCH, Vice Chancellor, B.A., M.A. Ed.D. ........................................ New York SAUL B. COHEN, B.A., M.A., Ph.D. ................................................................... New Rochelle JAMES C. DAWSON, A.A., B.A., M.S., Ph.D. ....................................................... Peru ANTHONY S. BOTTAR, B.A., J.D. ......................................................................... Syracuse GERALDINE D. CHAPEY, B.A., M.A., Ed.D. ......................................................... Belle Harbor ARNOLD B. GARDNER, B.A., LL.B. ...................................................................... Buffalo HARRY PHILLIPS, 3rd, B.A., M.S.F.S. ................................................................... Hartsdale JOSEPH E. BOWMAN,JR., B.A., M.L.S., M.A., M.Ed., Ed.D. ................................ Albany JAMES R. TALLON,JR., B.A., M.A. ...................................................................... Binghamton MILTON L. COFIELD, B.S., M.B.A., Ph.D. ........................................................... Rochester ROGER B. TILLES, B.A., J.D. ............................................................................... Great Neck KAREN BROOKS HOPKINS, B.A., M.F.A. ............................................................... Brooklyn NATALIE M. GOMEZ-VELEZ, B.A., J.D. ............................................................... -

Central Library of Rochester and Monroe County · Historic Monographs Collection
Central Library of Rochester and Monroe County · Historic Monographs Collection Central Library of Rochester and Monroe County · Historic Monographs Collection A FOB THE TOURIST J1ND TRAVELLER, ALONO THE LINE OF THE CANALS, AND TUB INTERIOli COMMERCE OF THE STATE OF NEW-YORK. BT HORATIO GATES SPAFFORD, LL. IX AUTHOR OF THE GAZETTEER Of SKW-IOBK. JfEW-YOBK: PRIXTEB BY T. AND J. SWORDS, No. 99 Pearl-street. 1824. Prfee SO Ceats. Central Library of Rochester and Monroe County · Historic Monographs Collection Northern-District of New-York, In wit: BE it remembered, thut on the twelfth day of July, in the forty-ninth year of the Inde pendence of the United States of America, A. D 1824. Harutio G. Spajford, of the said District, hath deposited in this Office the title of a Book, the right whereof he claims as Author, in the word& following, to wit: **A Pocket Guide for the Tourist and Traveller, along the line of the Canals, and the interior Commerce of the State of New-York. By Horatio Gates Spaffor'dyLL.D. Author of the Gazetteer of Nete-York." In conformity to the Act of the Congress of the United States, entitled, " An Act for the Encouragement of Learn ing, !>y securing the Copies of Maps, Charts, and Books, to the Authors and Proprietors of such Copies, during the times therein mentioned;" and also to the Act, entitled " An Act, supplementary to an Act, entitled ' An Act for the Encou ragement of Learning, !>y securing the Copies of Maps, Charts, and Hooks, to the Authors and Proprietors of such Copies during the times therein mentioned,' and extending the Benefits thereof to the Arts of Designing, Engraving, and Etching Historical and other Prints." R. -

Index of Surface-Water Records
~EOLOGICAL SURVEY CIRCULAR 138 July 1951 INDEX OF SURFACE-WATER RECORDS PART I.-NORTH ATLANTIC SLOPE BASINS TO SEPTEMBER 30, 1950 Prepared by Boston District UNITED STATES DEPARTMENT OF THE INTERIOR Oscar L. Chapman, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director Washington, 'J. C. Free on application to the Geological Survey, Washington 26, D. C. INDEX OF SURFACE-WATER RECORDS PART 1.-NORTH ATLANTIC SLOPE BASINS TO SEPTEMBER 30, 1950 EXPLANATION The index lists the stream-flow and reservoir stations in the North Atlantic Slope Basins for which records have been or are to be published for periods prior to Sept. 30, 1950. The stations are listed in downstream order. Tributary streams are indicated by indention. Station names are given in their most recently published forms. Parentheses around part of a station name indicate that the inclosed word or words were used in an earlier published name of the station or in a name under which records were published by some agency other than the Geological Survey. The drainage areas, in square miles, are the latest figures pu~lished or otherwise available at this time. Drainage areas that were obviously inconsistent with other drainage areas on the same stream have been omitted. Under "period of record" breaks of less than a 12-month period are not shown. A dash not followed immediately by a closing date shows that the station was in operation on September 30, 1950. The years given are calendar years. Periods·of records published by agencies other than the Geological Survey are listed in parentheses only when they contain more detailed information or are for periods not reported in publications of the Geological Survey. -

Post Office Box 1130, Cooperstown, NY 13326 · Tel: 607 547 8881 · Fax
The Honorable Andrew M. Cuomo Governor of New York State NYS State Capitol Building Albany, NY 12224 Joseph Martens, Commissioner Dr. Howard Zucker, Acting Commissioner NYS Department of Conservation NYS Department of Health 625 Broadway Corning Tower, Empire State Plaza Albany, NY 12233-1011 Albany, NY 12237 March 24, 2015 RE: Brookman Corners Compressor Station Dear Governor Cuomo, DEC Commissioner Martens, and DOH Acting Commissioner Zucker: Otsego 2000 is extremely concerned with plans by Dominion Transmission Inc. (DTI) to expand its Brookman Corners compressor station in Montgomery County, which would expose families and children in the vicinity to high levels of pollutants dangerous to human health. (FERC Docket #CP14-497-000) Please see the attached information that we provided to NYS-DEC project manager, Chris Hogan, in a meeting with him and his staff on March 19, 2015. During that meeting we discussed errors and misrepresentations by DTI, including erroneous air dispersion modeling which neglects impacts to the Otsquago Valley and village of Fort Plain. However we also recommended several design and development improvements, described herein, that could significantly reduce those emission levels and other impacts to the valley, including Otsquago Creek. Otsego 2000 maintains that this relevant and factual information should be reviewed before agencies consider air and water resource permits for this project. Respectfully, our objective is to encourage dialogue and avoid circumstances that often result in litigation, resentment of industry, and disillusion with government agencies entrusted to protect the public. Our organization seeks to achieve positive solutions whenever possible, so we urge your active support and involvement to facilitate a better outcome in these proceedings. -

Round 24 Agricultural Nonpoint Source Abatement and Control Program Project Descriptions All Projects Support the New York State
Round 24 Agricultural Nonpoint Source Abatement gricultural A and Control Program Project Descriptions Environmental All projects support the New York State Agricultural Management Environmental Management (AEM) Program by funding the implementation of agricultural water quality Best Management Practices (BMPs) to protect natural resources while maintaining the economic viability of New York State’s diverse agricultural community. Western NY $122,100 was awarded to the Allegany County Soil and Water Conservation District to work with two farms in the Wiscoy Creek Watershed. The project will: • Reduce nutrient and sediment loading within the watershed • Improve manure management on two farm facilities • Control livestock access to the stream with exclusionary fence and access control $572,204 was awarded to the Cattaraugus County Soil and Water Conservation District to work with one farm in the Cattaraugus Creek Watershed. The project will: • Reduce agricultural runoff and improve manure management • Implement a riparian buffer for additional water quality protection $779,900 was awarded to the Cattaraugus County Soil and Water Conservation District to work with three farms in the Conewango Creek Watershed. The project will: • Significantly help reduce the impact of runoff • Improve agricultural waste management • Establish riparian buffers in sensitive areas to help reduce sediment and phosphorus runoff. $156,255 was awarded to the Chautauqua County Soil and Water Conservation District to work with one farm in the Clymer Water District which includes Hulbert/Clymer Pond. The project will: • Reduce phosphorus and nitrogen inputs to the Clymer Aquifer and surface water resources • Improve agricultural waste management • Implement a riparian herbaceous buffer to filter nutrients and sediment from livestock pasture $809,370 was awarded to the Chautauqua County Soil and Water Conservation District to work with two farms in the Conewango Creek Watershed. -

Freeze-Up Ice Jams
ICE JAM REFERENCE AND TROUBLE SPOTS Ice Jam Reference Ice jams cause localized flooding and can quickly cause serious problems in the NWS Albany Hydrologic Service Area (HSA). Rapid rises behind the jams can lead to temporary lakes and flooding of homes and roads along rivers. A sudden release of a jam can lead to flash flooding below with the addition of large pieces of ice in the wall of water which will damage or destroy most things in its path. Ice jams are of two forms: Freeze up and Break up. Freeze up jams usually occur early to mid winter during extremely cold weather. Break up jams usually occur mid to late winter with thaws. NWS Albany Freeze Up Jam Criteria: Three Consecutive Days with daily average temperatures <= 0°F NWS Albany Break Up Jam Criteria: 1) Ice around 1 foot thick or more? And 2) Daily Average Temperature forecast to be >= 42°F or more? Daily Average Temperature = (Tmax+Tmin)/2 Rainfall/snowmelt with a thaw will enhance the potential for break up jams as rising water helps to lift and break up the ice. A very short thaw with little or no rain/snowmelt may not be enough to break up thick ice. ** River forecasts found at: http://water.weather.gov/ahps2/forecasts.php?wfo=aly will not take into account the effect of ice. ** Ice jams usually form in preferred locations in the NWS Albany HSA. See the “Ice Jam Trouble Spots” below for a list of locations where ice jams frequently occur. Ice Jam Trouble Spots **This is not an all inclusive list, but rather a list of locations where ice jams have been reported in the past.