Itli Lawrence Berkeley Laboratory .-;:I UNIVERSITY of CALIFORNIA CHEMICAL BIODYNAMICS DIVISION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzymatic Encoding Methods for Efficient Synthesis Of
(19) TZZ__T (11) EP 1 957 644 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/10 (2006.01) C12Q 1/68 (2006.01) 01.12.2010 Bulletin 2010/48 C40B 40/06 (2006.01) C40B 50/06 (2006.01) (21) Application number: 06818144.5 (86) International application number: PCT/DK2006/000685 (22) Date of filing: 01.12.2006 (87) International publication number: WO 2007/062664 (07.06.2007 Gazette 2007/23) (54) ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES ENZYMVERMITTELNDE KODIERUNGSMETHODEN FÜR EINE EFFIZIENTE SYNTHESE VON GROSSEN BIBLIOTHEKEN PROCEDES DE CODAGE ENZYMATIQUE DESTINES A LA SYNTHESE EFFICACE DE BIBLIOTHEQUES IMPORTANTES (84) Designated Contracting States: • GOLDBECH, Anne AT BE BG CH CY CZ DE DK EE ES FI FR GB GR DK-2200 Copenhagen N (DK) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • DE LEON, Daen SK TR DK-2300 Copenhagen S (DK) Designated Extension States: • KALDOR, Ditte Kievsmose AL BA HR MK RS DK-2880 Bagsvaerd (DK) • SLØK, Frank Abilgaard (30) Priority: 01.12.2005 DK 200501704 DK-3450 Allerød (DK) 02.12.2005 US 741490 P • HUSEMOEN, Birgitte Nystrup DK-2500 Valby (DK) (43) Date of publication of application: • DOLBERG, Johannes 20.08.2008 Bulletin 2008/34 DK-1674 Copenhagen V (DK) • JENSEN, Kim Birkebæk (73) Proprietor: Nuevolution A/S DK-2610 Rødovre (DK) 2100 Copenhagen 0 (DK) • PETERSEN, Lene DK-2100 Copenhagen Ø (DK) (72) Inventors: • NØRREGAARD-MADSEN, Mads • FRANCH, Thomas DK-3460 Birkerød (DK) DK-3070 Snekkersten (DK) • GODSKESEN, -

Nanor:Noles C/Mg Protein
Lawrence Berkeley National Laboratory Recent Work Title INTERRELATIONSHIP OF GLYCOGEN METABOLISM AND LACTOSE SYNTHESIS IN MAMMARY EPITHELIAL CELLS OF MICE Permalink https://escholarship.org/uc/item/2r05952r Author Emerman, J.T. Publication Date 1979-10-01 eScholarship.org Powered by the California Digital Library University of California LBL-9894~. d. {/ Preprint ITlI Lawrence Berkeley Laboratory .-;:I UNIVERSITY OF CALIFORNIA CHEMICAL BIODYNAMICS DIVISION Submitted to the Journal of Biological Chemistry -- INTERRELATIONSHIP OF GLYCOGEN METABOLISM AND LACTOSE SYNTHESIS IN MAMMARY EPITHELIAL CELLS OF MICE Joanne .T. Emerman, Jack C. Bartley, and Mina J. Bissell October 1979 RECEIVED LAWRENCE TWO-W E,K LOAN COpy LIBRARY )lIND DOCUMENT.S SEeT!G This is a Library Circulatin9 Copy which may be borrowed for two weeks. For a personal retention" ~, copy, call Tech. Info. Diuision, "Ext. .. 6782 . - "'; . , f.·l~ " /(, Prepared for the U.S. Department of Energy under Contract W-7405-ENG-48 DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. -

(12) STANDARD PATENT (11) Application No. AU 2015215937 B2 (19) AUSTRALIAN PATENT OFFICE
(12) STANDARD PATENT (11) Application No. AU 2015215937 B2 (19) AUSTRALIAN PATENT OFFICE (54) Title Metabolically engineered organisms for the production of added value bio-products (51) International Patent Classification(s) C12N 15/52 (2006.01) C12P 19/26 (2006.01) C12P 19/18 (2006.01) C12P 19/30 (2006.01) (21) Application No: 2015215937 (22) Date of Filing: 2015.08.21 (43) Publication Date: 2015.09.10 (43) Publication Journal Date: 2015.09.10 (44) Accepted Journal Date: 2017.03.16 (62) Divisional of: 2011278315 (71) Applicant(s) Universiteit Gent (72) Inventor(s) MAERTENS, Jo;BEAUPREZ, Joeri;DE MEY, Marjan (74) Agent / Attorney Griffith Hack, GPO Box 3125, Brisbane, QLD, 4001, AU (56) Related Art Trinchera, M. et al., 'Dictyostelium cytosolic fucosyltransferase synthesizes H type 1 trisaccharide in vitro', FEBS Letters, 1996, Vol. 395, pages 68-72 GenBank accession no. AF279134, 6 May 2002 van der Wel, H. et al., 'A bifunctional diglycosyltransferase forms the Fuc#1,2Gal#1,3-disaccharide on Skpl in the cytoplasm of Dictyostelium', The Journal of Biological Chemistry, 2002, Vol. 277, No. 48, pages 46527-46534 WO 2010/070104 Al Abstract The present invention relates to genetically engineered organisms, especially microorganisms such as bacteria and yeasts, for the production of added value bio-products such as specialty saccharide, activated saccharide, nucleoside, glycoside, glycolipid or glycoprotein. More specifically, the present invention relates to host cells that are metabolically engineered so that they can produce said valuable specialty products in large quantities and at a high rate by bypassing classical technical problems that occur in biocatalytical or fermentative production processes. -

Epithelial Cells of Mice
Biochem. J. (1980) 192, 695-702 695 Printed in Great Britain Interrelationship of glycogen metabolism and lactose synthesis in mammary epithelial cells of mice Joanne T. EMERMAN*, Jack C. BARTLEYt and Mina J. BISSELL Laboratory ofChemical Biodynamics, Lawrence Berkeley Laboratory, University ofCalifornia, Berkeley, CA 94720, U.S.A.t (Received 9 May 1980/Accepted 21 July 1980) Glycogen metabolism in mammary epithelial cells was investigated (i) by studying the conversion of glucose into glycogen and other cellular products in these cells from virgin, pregnant and lactating mice and (ii) by assaying the enzymes directly involved with glycogen metabolism. We find that: (1) mammary epithelial cells synthesized glycogen at rates up to over 60% that of the whole gland; (2) the rate of this synthesis was modulated greatly during the reproductive cycle, reaching a peak in late pregnancy and decreasing rapidly at parturition, when abundant synthesis of lactose was initiated; (3) glycogen synthase and phosphorylase activities reflected this modulation in glycogen metabolism; (4) lactose synthesis reached a plateau during late pregnancy, even though lactose synthase is reported to increase in the mouse mammary gland at this time. We propose that glycogen synthesis restricts lactose synthesis during late pregnancy by competing successfully for the shared UDP-glucose pool. The physiological advantage of glycogen accumulation during late pregnancy is discussed. The significance of glycogen metabolism in possibly because the cultures were known to be mammary epithelial cells has not been considered contaminated with other types of cells. seriously. Putative glycogen granules have been In a preliminary study on the metabolic patterns identified in these cells in the dog (Sekri & Faulkin, of the mammary gland at different stages of the 1967), cow (Reid & Chandler, 1973) and human reproductive cycle, we found that glands from (Sterling & Chandler, 1977). -

Building a Glycosylation Platform in E. Co/I Through Metabolic Engineering Frederik De Bruyn
Building a Glycosylation Platform in E. co/i through Metabolic Engineering Frederik De Bruyn Examination committee Prof. dr. ir. Paul Van Der Meeren (Ghent University, Chair) Prof. dr. ir. Matthias D'hooghe (Ghent University) Prof. dr. Magda Faijes (Universitat Ramon Llull, Spain) Prof. dr. ir. Els Van Damme (Ghent University) dr. ir. Manu De Groeve (Ablynx) Prof. dr. ir. Wim Soetaert (Ghent University) Prof. dr. ir. Marjan De Mey (Ghent University) Supervisors Prof. dr. ir. Wim Soetaert (Ghent University) Prof. dr. ir. Marjan De Mey (Ghent University) Department of Biochemical and Microbial Technology, Center of Expertise – Industrial Biotechnology and Biocatalysis, Ghent University Dean Prof. dr. ir. Guido Van Huylenbroeck Rector Prof. dr. Anne De Paepe Ghent University Faculty of Bioscience Engineering Department of Biochemical and Microbial Technology Building a Glycosylation Platform in E. coli through Metabolic Engineering Frederik De Bruyn Thesis submitted for the fulfillment of the requirements For the degree of Doctor (PhD) in Applied Biological Sciences Academic year: 2014-2015 Dutch translation of the title: Ontwikkeling van een glycosyleringsplatform in E. coli door Metabolic Engineering To refer to this thesis: De Bruyn, F. (2014) Building a Glycosylation Platform in E. coli through Metabolic Engineering. PhD thesis, Faculty of Bioscience Engineering, Ghent University, Ghent. Cover illustration: ©iStockphoto.com Edited by Brecht De Paepe and Frederik De Bruyn ISBN 978-90-5989-744-1 Copyright © 2014 by Frederik De Bruyn. All rights reserved The author and the promotors give the authorization to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. -

Milk Biosynthesis
FairLife: Cold Filtration • https://www.youtube.com/watch?v=YgHFaNVofY8 Milk Biosynthesis Part 5: Lactose, Mineral, and Vitamin Secretion Lactose is… • The major carbohydrate in milk • Present in milk of most mammals • A readily digestible source of glucose for the neonate • Digested by lactase enzyme in neonate • Unique to the mammary gland • The major osmoregulator of milk (draws water into gland) Lactose Concentration • Least variable milk component • High lactose concentration will not exist • More lactose = more water coming into alveolar lumen keeping lactose concentration fairly constant • Low lactose concentration will occur during involution and some illnesses • Remember milk is the only source of water for a neonate so lactose is very important!! What is lactose? • Over 60% of whole body glucose use in the lactating dairy cow is for lactose synthesis • Lactose = 1 molecule glucose + 1 molecule galactose • Glucose alone is the primary substrate for lactose synthesis • One glucose is converted to UDP-glucose, which is converted to one UDP-galactose • Another glucose is used for lactose synthesis without modification • 2 glucoses are required for each lactose molecule synthesized Glucose • Glucose passes across the Golgi membrane into the Golgi lumen by a glucose transporter (GLUT 1) • Presence of GLUT 1 on the Golgi membrane is specific to the mammary epithelial cell • Glucose transport does not require energy, but it is affected by glucose levels in cytoplasm Lactose Synthesis • UDP-galactose is actively transported into the -

Ep 1 117 822 B1
Europäisches Patentamt (19) European Patent Office & Office européen des brevets (11) EP 1 117 822 B1 (12) EUROPÄISCHE PATENTSCHRIFT (45) Veröffentlichungstag und Bekanntmachung des (51) Int Cl.: Hinweises auf die Patenterteilung: C12P 19/18 (2006.01) C12N 9/10 (2006.01) 03.05.2006 Patentblatt 2006/18 C12N 15/54 (2006.01) C08B 30/00 (2006.01) A61K 47/36 (2006.01) (21) Anmeldenummer: 99950660.3 (86) Internationale Anmeldenummer: (22) Anmeldetag: 07.10.1999 PCT/EP1999/007518 (87) Internationale Veröffentlichungsnummer: WO 2000/022155 (20.04.2000 Gazette 2000/16) (54) HERSTELLUNG VON POLYGLUCANEN DURCH AMYLOSUCRASE IN GEGENWART EINER TRANSFERASE PREPARATION OF POLYGLUCANS BY AMYLOSUCRASE IN THE PRESENCE OF A TRANSFERASE PREPARATION DES POLYGLUCANES PAR AMYLOSUCRASE EN PRESENCE D’UNE TRANSFERASE (84) Benannte Vertragsstaaten: (56) Entgegenhaltungen: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU WO-A-00/14249 WO-A-00/22140 MC NL PT SE WO-A-95/31553 (30) Priorität: 09.10.1998 DE 19846492 • OKADA, GENTARO ET AL: "New studies on amylosucrase, a bacterial.alpha.-D-glucosylase (43) Veröffentlichungstag der Anmeldung: that directly converts sucrose to a glycogen- 25.07.2001 Patentblatt 2001/30 like.alpha.-glucan" J. BIOL. CHEM. (1974), 249(1), 126-35, XP000867741 (73) Patentinhaber: Südzucker AG Mannheim/ • BUTTCHER, VOLKER ET AL: "Cloning and Ochsenfurt characterization of the gene for amylosucrase 68165 Mannheim (DE) from Neisseria polysaccharea: production of a linear.alpha.-1,4-glucan" J. BACTERIOL. (1997), (72) Erfinder: 179(10), 3324-3330, XP002129879 • GALLERT, Karl-Christian • DE MONTALK, G. POTOCKI ET AL: "Sequence D-61184 Karben (DE) analysis of the gene encoding amylosucrase • BENGS, Holger from Neisseria polysaccharea and D-60598 Frankfurt am Main (DE) characterization of the recombinant enzyme" J. -

O O2 Enzymes Available from Sigma Enzymes Available from Sigma
COO 2.7.1.15 Ribokinase OXIDOREDUCTASES CONH2 COO 2.7.1.16 Ribulokinase 1.1.1.1 Alcohol dehydrogenase BLOOD GROUP + O O + O O 1.1.1.3 Homoserine dehydrogenase HYALURONIC ACID DERMATAN ALGINATES O-ANTIGENS STARCH GLYCOGEN CH COO N COO 2.7.1.17 Xylulokinase P GLYCOPROTEINS SUBSTANCES 2 OH N + COO 1.1.1.8 Glycerol-3-phosphate dehydrogenase Ribose -O - P - O - P - O- Adenosine(P) Ribose - O - P - O - P - O -Adenosine NICOTINATE 2.7.1.19 Phosphoribulokinase GANGLIOSIDES PEPTIDO- CH OH CH OH N 1 + COO 1.1.1.9 D-Xylulose reductase 2 2 NH .2.1 2.7.1.24 Dephospho-CoA kinase O CHITIN CHONDROITIN PECTIN INULIN CELLULOSE O O NH O O O O Ribose- P 2.4 N N RP 1.1.1.10 l-Xylulose reductase MUCINS GLYCAN 6.3.5.1 2.7.7.18 2.7.1.25 Adenylylsulfate kinase CH2OH HO Indoleacetate Indoxyl + 1.1.1.14 l-Iditol dehydrogenase L O O O Desamino-NAD Nicotinate- Quinolinate- A 2.7.1.28 Triokinase O O 1.1.1.132 HO (Auxin) NAD(P) 6.3.1.5 2.4.2.19 1.1.1.19 Glucuronate reductase CHOH - 2.4.1.68 CH3 OH OH OH nucleotide 2.7.1.30 Glycerol kinase Y - COO nucleotide 2.7.1.31 Glycerate kinase 1.1.1.21 Aldehyde reductase AcNH CHOH COO 6.3.2.7-10 2.4.1.69 O 1.2.3.7 2.4.2.19 R OPPT OH OH + 1.1.1.22 UDPglucose dehydrogenase 2.4.99.7 HO O OPPU HO 2.7.1.32 Choline kinase S CH2OH 6.3.2.13 OH OPPU CH HO CH2CH(NH3)COO HO CH CH NH HO CH2CH2NHCOCH3 CH O CH CH NHCOCH COO 1.1.1.23 Histidinol dehydrogenase OPC 2.4.1.17 3 2.4.1.29 CH CHO 2 2 2 3 2 2 3 O 2.7.1.33 Pantothenate kinase CH3CH NHAC OH OH OH LACTOSE 2 COO 1.1.1.25 Shikimate dehydrogenase A HO HO OPPG CH OH 2.7.1.34 Pantetheine kinase UDP- TDP-Rhamnose 2 NH NH NH NH N M 2.7.1.36 Mevalonate kinase 1.1.1.27 Lactate dehydrogenase HO COO- GDP- 2.4.1.21 O NH NH 4.1.1.28 2.3.1.5 2.1.1.4 1.1.1.29 Glycerate dehydrogenase C UDP-N-Ac-Muramate Iduronate OH 2.4.1.1 2.4.1.11 HO 5-Hydroxy- 5-Hydroxytryptamine N-Acetyl-serotonin N-Acetyl-5-O-methyl-serotonin Quinolinate 2.7.1.39 Homoserine kinase Mannuronate CH3 etc. -
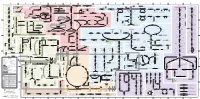
Fullsubwaymap221.Pdf
A B C D E F G H I J K L M Aldose reductase Sorbitol dehydrogenase Cholesterol synthesis Glycogen and galactose metabolism (branch point only) (debranching) glucose sorbitol fructose Aromatic amino acid metabolism Purine synthesis Pyrimidine synthesis glycogen (n+1) phenylacetate triiodothyronine (T3) dopaquinone melanin + + thyroxine (T4) (multiple steps) (many cycles) P 4' NADPH NADP NAD NADH acetyl-CoA x2 i Debranching enzyme 3' - α-1,4 bonds Aromatic amino acid 5-phosphoribosyl pyrophosphate (PRPP) HCO B 2' P 3 (branching) Glycogen 6 i (multiple steps) O H O (multiple steps) Tyrosinase O H O 1' 2 2 2 2 B 1 2 3 4 5 6 7 8 9 10 Pentose phosphate pathway Phenylalanine Tyrosine decarboxylase 6 Dopamine B Thiolase phosphorylase -5 -4 -3 -2 -1 0 1 2 3 4 Transaminase 6 glutamine 2 ATP (many cycles) Glycogen Glucose-6-phosphatase Dehydrogenase hydroxylase hydroxylase (DOPA decarboxylase) β-hydroxylase Methyltransferase 10 UDP 4:4 Transferase ATP glutathione (reduced) H O phenyllactate phenylpyruvate phenylalanine tyrosine dihydroxy- dopamine Glutamine PRPP CoA synthase Hexokinase or (in endoplasmic reticulum) 2 2 norepinephrine epinephrine glutamine Carbamoyl 9 1' phenylanine amidotransferase (GPAT) glutamate glycogen (n) Glutathione reductase Glutathione peroxidase + phosphate glycogen (n) -5 -4 -3 -2 -1 0 1 2 3 4 2' 3' 4' glucokinase 8 NADP NADPH glutamate α-ketoglutarate CO2 O H O Glycosyl (4:6) UDP-glucose ADP (L-DOPA) 2 2 synthetase II glutathione (oxidized) 2 H O α-ketoglutarate PPi glutamate acetoacetyl-CoA 7 1 Glucose -1,6-Glucosidase -

Ep 3235907 A1
(19) TZZ¥ ¥Z_T (11) EP 3 235 907 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 25.10.2017 Bulletin 2017/43 C12N 15/52 (2006.01) C12P 19/18 (2006.01) C12P 19/26 (2006.01) C12P 19/30 (2006.01) (21) Application number: 17169628.9 (22) Date of filing: 12.07.2011 (84) Designated Contracting States: • Beauprez, Joeri AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 8450 Bredene (BE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • De Mey, Marjan PL PT RO RS SE SI SK SM TR 9000 Gent (BE) (30) Priority: 12.07.2010 EP 10169304 (74) Representative: De Clercq & Partners Edgard Gevaertdreef 10a (62) Document number(s) of the earlier application(s) in 9830 Sint-Martens-Latem (BE) accordance with Art. 76 EPC: 11739017.9 / 2 593 549 Remarks: This application was filed on 05.05.2017 as a (71) Applicant: Universiteit Gent divisional application to the application mentioned 9000 Gent (BE) under INID code 62. (72) Inventors: • Maertens, Jo 9000 Gent (BE) (54) METABOLICALLY ENGINEERED ORGANISMS FOR THE PRODUCTION OF ADDED VALUE BIO-PRODUCTS (57) The present invention relates to genetically en- cells that are metabolically engineered so that they can gineered organisms, especially microorganisms such as produce said valuable specialty products in large quan- bacteria and yeasts, for the production of added value tities and at a high rate by bypassing classical technical bio-products suchas specialty saccharide, activated sac- problems that occur in biocatalytical or fermentative pro- charide, nucleoside,glycoside, glycolipid or glycoprotein. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0165635 A1 Copenhaver Et Al
US 2011 O165635A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0165635 A1 Copenhaver et al. (43) Pub. Date: Jul. 7, 2011 (54) METHODS AND MATERALS FOR Publication Classification PROCESSINGA FEEDSTOCK (51) Int. Cl. CI2P I 7/04 (2006.01) (75) Inventors: Gregory P. Copenhaver, Chapel CI2P I/00 (2006.01) Hill, NC (US); Daphne Preuss, CI2P 7/04 (2006.01) Chicago, IL (US); Jennifer Mach, CI2P 7/16 (2006.01) Chicago, IL (US) CI2P 7/06 (2006.01) CI2P 5/00 (2006.01) CI2P 5/02 (2006.01) (73) Assignee: CHROMATIN, INC., Chicago, IL CI2P3/00 (2006.01) (US) CI2P I/02 (2006.01) CI2N 5/10 (2006.01) (21) Appl. No.: 12/989,038 CI2N L/15 (2006.01) CI2N I/3 (2006.01) (52) U.S. Cl. ........... 435/126; 435/41; 435/157; 435/160; (22) PCT Fled: Apr. 21, 2009 435/161; 435/166; 435/167; 435/168; 435/171; 435/419,435/254.11: 435/257.2 (86) PCT NO.: PCT/US2O09/041260 (57) ABSTRACT S371 (c)(1), The present disclosure relates generally to methods for pro (2), (4) Date: Mar. 11, 2011 cessing a feedstock. Specifically, methods are provided for processing a feedstock by mixing the feedstock with an addi tive organism that comprises one or more transgenes coding Related U.S. Application Data for one or more enzymes. The expressed enzymes may be (60) Provisional application No. 61/046,705, filed on Apr. capable of breaking down cellulosic and lignocellulosic 21, 2008. materials and converting them to a biofuel.