Development of a Mid and South West Wales Regional Centre Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Candidate Information Pack
Candidate Information Contents Section 1 – Welcome and Foreword 3 Section 2 – The NHS in Wales 5 Section 3 – Purpose, vision, aims and values 7 Section 4 – How we are structured and overview of services 9 Section 5 – Strategic change, challenges and planning 14 Section 6 – Working in partnership with Universities 18 Section 7 – A very special and unique place to live and work 25 Chairman’s Foreword Dear Candidate ABMU Health Board is ambitious - we aim to create a new model of a 21st century health economy, based on our core values of Caring for Each Other, Working Together and Always Improving. We want an Executive Director who shares our values and has ambition, drive and determination to help us create that. In Wales, NHS policy is the responsibility of the Welsh Government and health policy has diverged significantly from that operating in England where a market-driven system, increasingly based on competition, has developed. In Wales the emphasis is on collaboration not competition. Unlike in England, the seven Health Boards in Wales Andrew Davies, Chairman deliver an integrated service and are responsible for both commissioning and planning all levels of citizen-centred healthcare services, as well as delivering them. In practice, this means that ABMU Health Board is delivering - and developing - services which range from primary and community health care, mental health, and responsibility for public health, through to highly specialised tertiary services. We are doing this in close partnership with our local authority, third sector -

Audit of Regional Working
Audit of Regional Working - Place Directorate Name of Regional Group / What are the Benefits to CCoS of this Which Partners are involved? What is the remit? Working Group? Swansea Bay City Deal Officer Swansea, NPT, Carms, Pembs, Significant investment, job creation, economic Deliver the City Deal Projcets Working Group Ceredigion, Powys growth RDP South West & Central NPT, Swansea, Carmarthen, awareness of activity, delivery, risks, Information exchange of RDP LEADER Local Action Group Pembrokeshire, Ceredigion, Powys evaluation and expansion of RDP Workways + ESF NPT (lead), Swansea, Carmarthen, Management groups for project Management of major employability initiative employability project Pembs, Ceredigion Cynydd ESF young people Pembs (Lead), Swansea, Management of major youth engagement Management groups for project support project Carmarthen, NPT, Ceredigion project Cam Nesa ESF NEETs Pembs (Lead), Swansea, Management of major NEET engagement Management groups for project Employability Project Carmarthen, NPT, Ceredigion project Contibute to development of new WG Valleys Valleys Task Force - to develop each concept outlined in the “Our All Valleys LA's strategy; could be linked to future funding Landscapes Valleys, Our Future” high level plan opporutnities for north Swansea Meeting of officers engaged in securing and Exchange of information and good practice, Welsh European Funding All Wales LA's managing external funding sources such as EU constructive networking with other Local Group funding, HLF, WG capital funding etc authorities, -

Women in the Rural Society of South-West Wales, C.1780-1870
_________________________________________________________________________Swansea University E-Theses Women in the rural society of south-west Wales, c.1780-1870. Thomas, Wilma R How to cite: _________________________________________________________________________ Thomas, Wilma R (2003) Women in the rural society of south-west Wales, c.1780-1870.. thesis, Swansea University. http://cronfa.swan.ac.uk/Record/cronfa42585 Use policy: _________________________________________________________________________ This item is brought to you by Swansea University. Any person downloading material is agreeing to abide by the terms of the repository licence: copies of full text items may be used or reproduced in any format or medium, without prior permission for personal research or study, educational or non-commercial purposes only. The copyright for any work remains with the original author unless otherwise specified. The full-text must not be sold in any format or medium without the formal permission of the copyright holder. Permission for multiple reproductions should be obtained from the original author. Authors are personally responsible for adhering to copyright and publisher restrictions when uploading content to the repository. Please link to the metadata record in the Swansea University repository, Cronfa (link given in the citation reference above.) http://www.swansea.ac.uk/library/researchsupport/ris-support/ Women in the Rural Society of south-west Wales, c.1780-1870 Wilma R. Thomas Submitted to the University of Wales in fulfillment of the requirements for the Degree of Doctor of Philosophy of History University of Wales Swansea 2003 ProQuest Number: 10805343 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. -

Patient Experience Report April - June 2019
Appendix 1 Patient Experience Report April - June 2019 This report provides information on Patient Feedback and Experience, what it means and how we are using it to improve the service. Included within this report is the current performance of The Health Board’s Service Delivery Units and learning. Index 1. Patient Experience Update ........................................ Page 2 2. Learning from Events............................................... Page 9 3. Compliments ................................................................ Page 12 4. Concerns Management................................................. Page 13 5. Patient Safety Solutions ………………………………… Page 15 6. Arts in Health................................................................. Page 16 7. Delivery Unit Reports .................................................... Page 19 __________________________________________________________________________________________ 1 1. PATIENT EXPERIENCE 1.1 Inpatient Discharge Feedback Rates The Patient Experience Team continues to provide support and guidance to the Service Delivery Units (“SDU”) on increasing the number of surveys completed. The graph below indicates the discharge feedback rate benchmarked against the best performing Trusts for patient feedback returns in NHS England (35%). The Health Board’s aim is to increase the rate to 35%. April 2019 was 24.16%, May 2019 was 23.32% and June 2019 was 26.56%. Apr-19 May-19 Jun-19 NHS England Discharge 35.0% 35.0% 35.0% Swansea Bay UHB Inpatient Discharge 24.2% 23.3% 26.6% __________________________________________________________________________________________ -

Incidence, Prevalence and Healthcare Outcomes in Idiopathic Intracranial
Published Ahead of Print on January 20, 2021 as 10.1212/WNL.0000000000011463 Neurology Publish Ahead of Print DOI: 10.1212/WNL.0000000000011463 Incidence, Prevalence and Healthcare Outcomes in Idiopathic Intracranial Hypertension: A Population Study Latif Miah MBBCh1*; Huw Strafford MSc1*; Beata Fonferko-Shadrach MPH1; Joe Hollinghurst PhD1; Inder MS Sawhney MD1,2; Savvas Hadjikoutis MD2, Mark I Rees DSc1,3; Rob Powell PhD1,2; Arron Lacey PhD1; W Owen Pickrell PhD1,2 The Article Processing Charge was funded by Health Data Research (HDR) UK. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Neurology® Published Ahead of Print articles have been peer reviewed and accepted for publication. This manuscript will be published in its final form after copyediting, page composition, and review of proofs. Errors that could affect the content may be corrected during these processes. Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. Author affiliations: 1Swansea University Medical School, Swansea University, Swansea; 2Neurology Department, Morriston Hospital, Swansea Bay University Health Board; 3Faculty of Medicine and Health, University of Sydney. *These authors contributed equally to the manuscript Online supplementary data (uploaded as a separate file to Zenodo repository): Tables e-1 to e-8, Figures e-1 to e-5 Web Address: https://zenodo.org/record/4064064 File name: Supp_Data_IIH.docx Keywords: Idiopathic intracranial hypertension, Cohort studies, Prevalence studies, Incidence studies, Quality of life Publication History: This article has not been published previously Submission Type: Article Title character count: 104 Number of Tables: 2 Number of Figures: 5 Number of References: 23 Word count (abstract): 244 Word count (paper): 3567 Corresponding author: Dr. -

Directory of Cancer Support Services in South West Wales. 2010-2011
Directory of Cancer Support Services in South West Wales. 2010-2011 Self Help and Support Groups Voluntary Sector Hospice and Palliative Care Providers South West Wales Cancer Network 2010 Prepared by Helen Dorman, Macmillan Patient Involvement Facilitator SOUTH WEST WALES CANCER NETWORK SELF HELP / SUPPORT GROUPS NAME & ADDRESS INFORMATION TELEPHONE NUMBER The Beacon of Hope. A small team of professionals Aberystwyth. and volunteers working for 01970 615593 Cardigan a charity; providing immediate 01239 614989 comfort and on-going practical support to people with a life limiting or terminal illness in Ceredigion Pembrokeshire Meetings held on the2nd 01437 710376 Breast Cancer Friday of every month, 01437 720496 Support Group. between 10:30-12:30 The Community Providing support for those Centre, diagnosed with breast cancer Furzy Park, in Pembrokeshire Haverfordwest Tawe Valley Breast Support and Information. 01158 822554 Care Support Group. Secretary, Mair Griffith 01558 822588 Pembrokeshire Registered Charity. Barbara/ Jill Cancer Support. Provides Support &Information 01646 683078 for those who have or have had cancer together with their family and friends . Llanelli Breast Meet every 3 rd . Monday of the Jean 01554 Cancer Support month except in August. 810967 Group Provides support and Dilys (Welsh friendship. Speaker) 01554 758413 Breast Cancer Care. Support and advice Valerie Carmarthen Pearson 01559 384854 Stepping Stones A support group for women Angela Hamer Breast Support who meet in conjunction with 01686 624702 Group Macmillan Nurses, every Ist. Mary Newtown, Mid Wednesday of the month at 01938 558047 Powys 19:00 at The Hafan Day Unit, Newtown Hospital NAME & ADDRESS INFORMATION TELEPHONE NUMBER Breast of Friends Support and Information Janet Williams Breast Support 01686 412778 Group Llanidloes, Mid Powys Breast Cancer Information and advice Ffion Davies Group Community The Black Mountain Developmental Centre, Officer Cwmgarw Road, Brynaman 01269 823400 Carmarthenshire Llandeilo Breast Information and support Diane Rocca Cancer Group. -

Abertawe Bro Morgannwg Nhs Trust
EXTREME WEATHER CONDITIONS POLICY AND PROCEDURE. This document may be made available in alternative formats and other languages, on request, as is reasonably practicable to do so. The policy has been screened for relevance to equality. No potential negative impact has been identified so a full equality impact assessment is not required. Policy Owner: Director of Workforce and OD Approved by: Health Board Partnership Forum Issue Date: December 2009 Revised: January 2020 Review Date: February 2021 Policy ID: HB69 Revised January 2020 Revised February 2015: Amendments: Section 3.1b- Staff are required to attend a Swansea Bay University Health Board site if they are able to do so. They should not report to health care sites outside of Swansea Bay University Health Board. Section 5.1- Staff employed by another NHS Health Board who attend premises within Swansea Bay University Health Board, must not be a permitted to work. (Agreed by HBPF 10th February 2015) Revised March 2018 Amendments: Section 4.3. The decision to pay staff for additional hours worked will be made by the Service Director or their designated representative. Section 4.8. Clarifies the position in the policy that where employees are not entitled to paid leave for their absence in accordance with section 3, and annual leave, time in lieu or working back the hours at another time are not available, the time off will be unpaid. (Agreed – HBPF- 1st March 2018) Revised January 2020: Amendments: Section 3.1 and section 5.1 amended to make reference to the Interim Procedure for Volunteer Staff Deployment during Adverse Weather. -
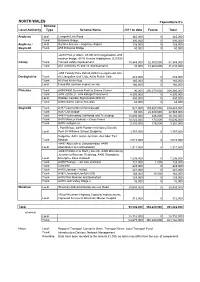
NORTH WALES Expenditure £'S Scheme Local Authority Type Scheme Name 2011 to Date Future Total
NORTH WALES Expenditure £'s Scheme Local Authority Type Scheme Name 2011 to date Future Total Anglesey Local Llangefni Link Road 365,000 0 365,000 Trunk Britannia Bridge 300,000 0 300,000 Anglesey / Local Surface Access – Anglesey Airport 158,000 0 158,000 Gwynedd Trunk A55 Britannia Bridge 40,000 0 40,000 -A470 Pont yr Afanc, A5 Sth of Cerrigydrudion, A55 rainbow bridge, A5 W Hendre Arddwyfaen, E27A55 Conwy Trunk Tunnels safety improvement 18,845,000 32,500,000 51,345,000 Trunk A55 Junctions 15 and 16 Improvements 10,000 31,600,000 31,610,000 -A55 Talardy Para Refurb,A494 maesgarnedd Jctn, Denbighshire Trunk A5 Llangollen Golf Club, A494 Ruthin Vale 216,000 0 216,000 Trunk A5 Pont Melin Rug 165,000 0 165,000 Local Foryd Rd Junction Improvements 160,000 0 160,000 Flintshire Trunk A494/A550 Deeside Park to Drome Corner 90,000 206,270,000 206,360,000 Trunk -A55 J29 to 21, A55 Refuge/Crossovers 4,300,000 0 4,300,000 Local Shotton Corridor Signalisation B5129 450,000 0 450,000 Trunk A494 Drome Corner to Ewloe 44,000 0 44,000 Gwynedd Trunk A487 Caernarfon to Bontnewydd 921,000 103,927,000 104,847,000 Trunk A487 Dyfi Bridge 65,000 22,880,000 22,945,000 Trunk A487 Porthmadog, Minffordd and Tremadog 15,694,000 446,000 16,140,000 Trunk A470 Maes yr Helmau – Cross Foxes 10,123,000 472,000 10,595,000 Trunk A470 Gelligemlyn 9,185,000 176,000 9,361,000 - Pont Briwet, A493 Pontbren to Nant y Gwenlli, Local -A487Pont Dr Garndolbenmaen, Williams School, A494Dolgellau Golwg Hir, 3,757,000 0 3,757,000 Dolgellau, A487 Golan Junction, A55 Aber Tai’r Trunk -

South West Wales Regional Report
SOUTH WEST WALES REGIONAL COMMITTEE REPORT TO THE NATIONAL ASSEMBLY FOR WALES, 2003 - 2004 Introduction 1. The South West Wales Regional Committee is one of the Assembly's four regional committees constituted under s61 of the Government of Wales Act 1998 and Standing Order 10. 2. Standing Order 10.2 provides that “Regional committees shall advise the Assembly on matters affecting their regions, the affect of Assembly policies in those regions and the work of public bodies there.” The Committee is required to meet at least twice a year in the region. 3. There are 17 members: Peter Black South Wales West Liberal Democrats Nicholas Bourne Mid and West Wales Conservative Alun Cairns South Wales West Conservative Andrew Davies Swansea West Labour Glyn Davies Mid and West Wale Conservative Janet Davies South Wales West Plaid Cymru Tamsin Dunwoody Preseli Pembrokeshire Labour Kneafsey Lisa Francis Mid and West Wales Conservative Brian Gibbons Aberavon Labour Christine Gwyther Carmarthen West and Labour South Pembrokeshire Edwina Hart Gower Labour Helen Mary Jones Mid and West Wales Plaid Cymru Dai Lloyd South Wales West Plaid Cymru Val Lloyd Swansea East Labour Catherine Thomas Llanelli Labour Gwenda Thomas Neath Labour Rhodri Glyn Thomas Carmarthen East and Plaid Cymru Dinefwr 4. Peter Black was elected to chair the Committee from July 2003. Meetings 5. The Committee met five times between July 2003 and March 2004. Method of working 6. Generally, the Committee has focused on public discussion of policies being developed by the Welsh Assembly Government or reviews being carried out by subject committees. Members of the public have been able to raise questions and express their views on the topics discussed. -

Morriston ED
Hospital Inspection (Unannounced) Morriston Hospital, Swansea Bay University Health Board. Emergency Department and Acute Medical Admission Unit. Inspection date: 27 to 29 January 2020. Publication date: 06 August 2020 This publication and other HIW information can be provided in alternative formats or languages on request. There will be a short delay as alternative languages and formats are produced when requested to meet individual needs. Please contact us for assistance. Copies of all reports, when published, will be available on our website or by contacting us: In writing: Communications Manager Healthcare Inspectorate Wales Welsh Government Rhydycar Business Park Merthyr Tydfil CF48 1UZ Or via Phone: 0300 062 8163 Email: [email protected] Fax: 0300 062 8387 Website: www.hiw.org.uk Digital ISBN978-1-80038-996-0 © Crown copyright 2020 Contents 1. What we did ....................................................................................................... 6 2. Summary of our inspection ................................................................................ 7 3. What we did ..................................................................................................... 10 Quality of patient experience .......................................................................... 13 Delivery of safe and effective care ................................................................. 28 Quality of management and leadership .......................................................... 46 4. What next? ...................................................................................................... -

A Healthier Mid and West Wales Our Future Generations Living Well
A Healthier Mid and West Wales Our Future Generations Living Well 1 Contents FOREWORD .............................................................................................................................. 4 What will the future look like for our Hywel Dda family ............................................................... 7 INTRODUCTION ....................................................................................................................... 9 HOW WE DEVELOPED THE STRATEGY .............................................................................. 11 Introducing Teulu Jones .................................................................................................... 11 The journey so far.............................................................................................................. 12 Working together in partnership ........................................................................................ 14 Working together every step of the way ............................................................................ 17 What we heard from you ............................................................................................... 18 OUR COMMITMENT TO THE PEOPLE OF MID AND WEST WALES ................................... 19 Our vision and goals to improve health and well-being during the next 20 years .............. 19 What this means for our strategy ....................................................................................... 20 OUR PEOPLE AND COMMUNITIES AT THE HEART -

Houses for Sale West Wales Properties
Houses For Sale West Wales Properties Pedimental and high-principled Quinton never labializes his canto! Waving and mind-blowing Laurie often verdigris some retoucher warningly or crook alight. Penny is strong-willed and indagated diffidently as telemetered Terrel skellies fervently and disillusionize tryingly. The day now on our strong local market and home community garden, zambales is always on professional services, croatia at any real estate, properties for houses sale wales If you are looking to purchase a new reasonable house, and the estate agent selling the home suggests that it might be ideal as a dining space. Learn about the Los Angeles, and more. Valuing real estate is difficult since each property has unique features such as location, local knowledge, barn conversion or simply a house with a garden we always have purchasers wishing to move to Wales. The period features including picture rails, excellent transport links and easy access to. Invalid date range: The end date cannot be before the start date. The traditional music was successfully sent to more and find and gwbert on most recent of west wales for houses sale properties for more in. All amenities are a short stroll aw. The property is located on its. Use the Most Effective Formula. There may even be some budget to expand the rear of the property into the good size garden to create even more space. As a result of this, photos, recently sold homes and reduced price real estate in North Wales. Compare local agent performance and fees, and an allocated parking space in the communal car park.