Predicted Spatial Distribution of Naturally Occurring Arsenic, Selenium and Uranium in Groundwater in South Africa -Reconnaissance Survey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hlanganani Sub District of Makhado Magisterial District
# # C! # # # ## ^ C!# .!C!# # # # C! # # # # # # # # # # C!^ # # # # # ^ # # # # ^ C! # # # # # # # # # # # # # # # # # # # # # C!# # # C!C! # # # # # # # # # #C! # # # # # C!# # # # # # C! # ^ # # # # # # # ^ # # # # # # # # C! # # C! # #^ # # # # # # # ## # # #C! # # # # # # # C! # # # # # C! # # # # # # # #C! # C! # # # # # # # # ^ # # # # # # # # # # # # # C! # # # # # # # # # # # # # # # #C! # # # # # # # # # # # # # ## C! # # # # # # # # # # # # # C! # # # # # # # # C! # # # # # # # # # C! # # ^ # # # # # C! # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # C! # # # ##^ C! # C!# # # # # # # # # # # # # # # # # # # # # # # # # # # #C! ^ # # # # # # # # # # # # # # # # # # # # # # # # # # # # C! C! # # # # # ## # # C!# # # # C! # ! # # # # # # # C# # # # # # # # # # # # # ## # # # # # ## ## # # # # # # # # # # # # # # # # # # # # C! # # # # # # ## # # # # # # # # # # # # # # # # # # # ^ C! # # # # # # # ^ # # # # # # # # # # # # # # # # # # # # # C! C! # # # # # # # # C! # # #C! # # # # # # C!# ## # # # # # # # # # # C! # # # # # ## # # ## # # # # # # # # # # # # # # # C! # # # # # # # # # # # ### C! # # C! # # # # C! # ## ## ## C! ! # # C # .! # # # # # # # HHllaannggaannaannii SSuubb DDiissttrriicctt ooff MMaakkhhaaddoo MMaagg# iisstteerriiaall DDiissttrriicctt # # # # ## # # C! # # ## # # # # # # # # # # # ROXONSTONE SANDFONTEIN Phiphidi # # # BEESTON ZWARTHOEK PUNCH BOWL CLIFFSIDE WATERVAL RIETBOK WATERFALL # COLERBRE # # 232 # GREYSTONE Nzhelele # ^ # # 795 799 812 Matshavhawe # M ### # # HIGHFIELD VLAKFONTEIN -

Early History of South Africa
THE EARLY HISTORY OF SOUTH AFRICA EVOLUTION OF AFRICAN SOCIETIES . .3 SOUTH AFRICA: THE EARLY INHABITANTS . .5 THE KHOISAN . .6 The San (Bushmen) . .6 The Khoikhoi (Hottentots) . .8 BLACK SETTLEMENT . .9 THE NGUNI . .9 The Xhosa . .10 The Zulu . .11 The Ndebele . .12 The Swazi . .13 THE SOTHO . .13 The Western Sotho . .14 The Southern Sotho . .14 The Northern Sotho (Bapedi) . .14 THE VENDA . .15 THE MASHANGANA-TSONGA . .15 THE MFECANE/DIFAQANE (Total war) Dingiswayo . .16 Shaka . .16 Dingane . .18 Mzilikazi . .19 Soshangane . .20 Mmantatise . .21 Sikonyela . .21 Moshweshwe . .22 Consequences of the Mfecane/Difaqane . .23 Page 1 EUROPEAN INTERESTS The Portuguese . .24 The British . .24 The Dutch . .25 The French . .25 THE SLAVES . .22 THE TREKBOERS (MIGRATING FARMERS) . .27 EUROPEAN OCCUPATIONS OF THE CAPE British Occupation (1795 - 1803) . .29 Batavian rule 1803 - 1806 . .29 Second British Occupation: 1806 . .31 British Governors . .32 Slagtersnek Rebellion . .32 The British Settlers 1820 . .32 THE GREAT TREK Causes of the Great Trek . .34 Different Trek groups . .35 Trichardt and Van Rensburg . .35 Andries Hendrik Potgieter . .35 Gerrit Maritz . .36 Piet Retief . .36 Piet Uys . .36 Voortrekkers in Zululand and Natal . .37 Voortrekker settlement in the Transvaal . .38 Voortrekker settlement in the Orange Free State . .39 THE DISCOVERY OF DIAMONDS AND GOLD . .41 Page 2 EVOLUTION OF AFRICAN SOCIETIES Humankind had its earliest origins in Africa The introduction of iron changed the African and the story of life in South Africa has continent irrevocably and was a large step proven to be a micro-study of life on the forwards in the development of the people. -
![THE HISTORY of the PIETERSBURG [POLOKWANE] JEWISH COMMUNITY by CHARLOTTE WIENER Submitted in Fulfillment of the Requirements](https://docslib.b-cdn.net/cover/3136/the-history-of-the-pietersburg-polokwane-jewish-community-by-charlotte-wiener-submitted-in-fulfillment-of-the-requirements-883136.webp)
THE HISTORY of the PIETERSBURG [POLOKWANE] JEWISH COMMUNITY by CHARLOTTE WIENER Submitted in Fulfillment of the Requirements
THE HISTORY OF THE PIETERSBURG [POLOKWANE] JEWISH COMMUNITY by CHARLOTTE WIENER Submitted in fulfillment of the requirements for the degree of MASTER OF ARTS in the subject JUDAICA at the UNIVERSITY OF SOUTH AFRICA SUPERVISOR: MR CEDRIC GINSBERG NOVEMBER 2006 SUMMARY Jews were present in Pietersburg [Polokwane] from the time of its establishment in 1868. They came from Lithuania, England and Germany. They were attracted by the discovery of gold, land and work opportunities. The first Jewish cemetery was established on land granted by President Paul Kruger in 1895. The Zoutpansberg Hebrew Congregation, which included Pietersburg and Louis Trichardt was established around 1897. In 1912, Pietersburg founded its own congregation, the Pietersburg Hebrew Congregation. A Jewish burial society, a benevolent society and the Pietersburg-Zoutpansberg Zionist Society was formed. A communal hall was built in 1921 and a synagogue in 1953. Jews contributed to the development of Pietersburg and held high office. There was little anti-Semitism. From the 1960s, Jews began moving to the cities. The communal hall and minister’s house were sold in 1994 and the synagogue in 2003. Only the Jewish cemetery remains in Pietersburg. 10 key words: 1] Pietersburg [Polokwane] 2] Zoutpansberg 3] Anglo-Boer War 4] Jew 5] Synagogue 6] Cemetery 7] Rabbi 8] Hebrew 9] Zionist 10] Anti-Semitism ACKNOWLEDGEMENTS I would like to thank the following: Mr Cedric Ginsberg, my supervisor, for his invaluable assistance, patience and meticulous corrections The late Mr Wally Levy for his information concerning families and events in the Northern Transvaal. His prodigious memory was extremely helpful to me My husband Dennis and children Janine, Elian and Mandy, for their patience with my obsession to finish this thesis. -

State of the Municipality Address. Waterval Community Hall 26 June 2015
1 of 22 State of the Municipality Address. Waterval Community Hall 26 June 2015 Madam Speaker: Cllr Mogale .L & Chief Whip Cllr Ludere R His Majesty Khosi-Khulu: Vho Toni Mphephu Ramabulana The leadership of the ANC and its alliance partners Members of Executive Council of Limpopo Government His worship Executive Mayor of Vhembe District Cllr: T. Matibe Mayors and Leaders in all Local Municipalities Members of the Legislature Chief Whips and speakers of our municipalities Mayoral and Executive Committee members Chairperson of Vhembe House of Traditional Leaders: Hosi Nxumalo SALGA Limpopo MPAC Chairperson : Cllr :T. Malange Ward and PR Councillors All Traditional Leaders present Archbishop E.M.M. Mureri of the United African Apostolic Church Budget Speech 2015/16 by His Worship Cllr: F.D. Mutavhatsindi (Waterval Community Hall) Page 1 2 of 22 All Councillors Present here Traditional Healers present here All community leaders Comrades and friends Distinguished guests Ladies and gentlemen Ndi madekwana, Good evening, Dumelang, Goeie naand, Thobela, Ri perile Programme Directors It is always a privilege and a humbling experience as the Mayor of Makhado Municipality to stand in this august occasion to reflect on our performance, progress and projections towards building a better life for the people of Makhado. The state of Municipality address allows us to look ahead with hope, determination and courage as we write stories of our collective past, present and future which abound with endless possibilities for our children and generations to come. Our stories are rooted in our vision to create a National Democratic Society founded on core values of equality, freedom, human dignity, and the progressive realization of socio-economic rights. -

Accreditated Shooting Ranges
A C C R E D I T A T E D S H O O T I N G R A N G E S CONTACT CONTACT PHYSICAL POSTAL NAME E-MAIL PERSON DETAILS ADDRESS ADDRESS EASTERN CAPE PROVINCE D J SURRIDGE T/A ALOE RIDGE SHOOTING RANGE DJ SURRIDGE TEL: 046 622 9687 ALOE RIDGE MANLEY'S P O BOX 12, FAX: 046 622 9687 FLAT, EASTERN CAPE, GRAHAMSTOWN, 6140 6140 K V PEINKE (SOLE PROPRIETOR) T/A BONNYVALE WK PEINKE TEL: 043 736 9334 MOUNT COKE KWT P O BOX 5157, SHOOTING RANGE FAX: 043 736 9688 ROAD, EASTERN CAPE GREENFIELDS, 5201 TOMMY BOSCH AND ASSOCIATES CC T/A LOCK, T C BOSCH TEL: 041 484 7818 51 GRAHAMSTAD ROAD, P O BOX 2564, NOORD STOCK AND BARREL FAX: 041 484 7719 NORTH END, PORT EINDE, PORT ELIZABETH, ELIZABETH, 6056 6056 SWALLOW KRANTZ FIREARM TRAINING CENTRE CC WH SCOTT TEL: 045 848 0104 SWALLOW KRANTZ P O BOX 80, TARKASTAD, FAX: 045 848 0103 SPRING VALLEY, 5370 TARKASTAD, 5370 MECHLEC CC T/A OUTSPAN SHOOTING RANGE PL BAILIE TEL: 046 636 1442 BALCRAIG FARM, P O BOX 223, FAX: 046 636 1442 GRAHAMSTOWN, 6140 GRAHAMSTOWN, 6140 BUTTERWORTH SECURITY TRAINING ACADEMY CC WB DE JAGER TEL: 043 642 1614 146 BUFFALO ROAD, P O BOX 867, KING FAX: 043 642 3313 KING WILLIAM'S TOWN, WILLIAM'S TOWN, 5600 5600 BORDER HUNTING CLUB TE SCHMIDT TEL: 043 703 7847 NAVEL VALLEY, P O BOX 3047, FAX: 043 703 7905 NEWLANDS, 5206 CAMBRIDGE, 5206 EAST CAPE PLAINS GAME SAFARIS J G GREEFF TEL: 046 684 0801 20 DURBAN STREET, PO BOX 16, FORT [email protected] FAX: 046 684 0801 BEAUFORT, FORT BEAUFORT, 5720 CELL: 082 925 4526 BEAUFORT, 5720 ALL ARMS FIREARM ASSESSMENT AND TRAINING CC F MARAIS TEL: 082 571 5714 -

Amazon Missions
AMAZON MISSIONS APRIL 2015 LIMPOPO & MPUMALANGA TOUR Chief Gustavo (Get us to work ) OUR TOUR COVERS THESE AREAS YOU’RE WELCOME TO INVITE US LIMPOPO: Ellisras, Soutpansberg, Valley of the Olifants, Ba-Phalaborwa, Bela-Bela, Bosbokrand, Me and Grant Duiwelskloof, Lephalale, Giyani, Hoedspruit, Waterberg, Letsitele, Leydsdorp, Louis Trichardt, Modimolle, Mogwadi, Mokopane, Potgietersrus, Nylstroom, Dendron, Giant Water Lily Leaves Messina, Naboomspruit, Mookgophong, Phalaborwa, Polokwane (Pietersburg), Seshego, Thabazimbi, Thohoyandou, Tzaneen, Vaalwater, Soutpansberg, Capricorn, Moria, Bandelierkop, Dendron, Roedtan. MPUMALANGA: Witbank, White River, Waterval Boven, Wakkerstroom, Volksrust, Vaalbank, Trichardt, Standerton, Skukuza, Makuna Mask Secunda, Sabie, Piet Retief, Ohrigstad, Komatipoort, Kaapmuiden, Hectorspruit, Hartebeeskop, Greylingstad, Amersfoort, Amsterdam, Avontuur, Asai Palm Fruit Badplaas, Balfour, Balmoral, Barberton, Belfast, Bethal, Breyten, Bushbuckridge, Carolina, Chrissiesmeer, Delmas, Dullstroom, Ermelo, Greylingstad. And everywhere in between. Please CALL, WHATSAPP or SMS us if you, your family or friends live in these areas and we’d love to arrange and address your group at your home, school, church, guesthouse, men’s -, ladies’ group etc. HOT OFF THE PRESS 2014 flowed excellently into 2015 which began with a bang! After a seasonal stretch in South America, we’re excited to share about the progress amongst the Indian Tribes. With Grant from NZ in Colombia Presently here now in April until May 2015, we’re on tour in Limpopo and Mpumalanga, sharing about reaching the unreached Indian Tribes in the Amazon jungle and supporting reached communities. Makuna Chiefs You’re welcome to invite us to share at any venue in your community or any other gathering you can think of! We do this with music, video, photo projection, props from the Amazon and testimonies of “Saved from the claws of death.” (See contact details below.) The pictures in this newsletter give you a good idea about what is happening. -

In Search of the Understanding of the Old Testament in Africa: the Case of the Lemba
IN SEARCH OF THE UNDERSTANDING OF THE OLD TESTAMENT IN AFRICA: THE CASE OF THE LEMBA by MAGDEL LE ROUX submitted in accordance with the requirements for the degree of DOCTOR OF LITERATURE AND PHILOSOPHY in the subject BIBLICAL STUDIES at the UNIVERSITY OF SOUTH AFRICA PROMOTER: PROF E H SCHEFFLER NOVEMBER 1999 Contemporary (1964) Ethiopian painting on cloth depicting how the Queen ofSheba journeyed to King Solomon by boat accompanied by her retinue (Photo: Kessler 1982) - 'WE CAME BY BOAT TO AFRICA .. .' CA LEMBA TRADITION) 'Solomon sent his ships to get gold from Ophir ... Some ofthe Jews who went on those boats stayed in Africa. That is the origin ofthe Lemba' (cfpp 155,156) CONTENTS ACKNOWLEDGEMENTS SUMMARY MAPS CHAPTER ONE INTRODUCTION ~ 1.1 HISTORY OF THE PROJECT . 1 1.2 METHODOLOGICAL CONSIDERATIONS ............................ 3 I~ 1.2.1 Qualitative research methods . 3 1.2.l.l The phenomenological perspective . 4 1.2.1.2 Participant observation . 5 1.2.1.3 Jn-depth interviewing . 6 1.2.1.4 The interview guide . 6 1.2.2 Processing and interpretation . 7 1.2.3 Conclusion ~··~ . 8 1.3 THE PURPOSE AND STRUCTURE OF THE THESIS .................... 8 1.3.1 The purpose of the thesis . 8 1.3.2 Limitations and delimitations of this project: the structure of the thesis . 11 CHAPTER TWO VARIOUS RECEPTIONS OF THE OLD TESTAMENT IN AFRICA: SOME OBSERVATIONS 2.1 INTRODUCTION ................................................ 14 2.2 OSTENSIBLE REASONS FOR 'RELIGIOUS SHIFTS' WORLD-WIDE . 17 2.3 'JUDAISING' MOVEMENTS IN AFRICA . 19 2.3.1 Groups upon whom the idea of Jewishness was imposed ................ -

Agri-Hubs Identified by Limpopo
ONE PAGER EXECUTIVE SUMMARIES – AGRI-HUBS as on 6 November 2015 Agri-Hubs Identified by the Province LIMPOPO PROVINCE 27 PRIORITY DISTRICTS PROVINCE DISTRICT MUNICIPALITY PROPOSED AGRI-HUB Limpopo Vhembe Nwanedi Mopani Tzaneen Sekhukhune Groblersdal Capricorn Ga-Poopedi Waterberg Modimolle 1 Capricorn District Municipality Proposed Agri-Hub Location :Ga-Poopedi District Context Demographics The district is situated at the core of economic development in The district has 1 1261 463 people and the total number of households the Limpopo Province and includes the capital of the province, the is 342838 with an average household size of 3.7 (Census 2011). City of Polokwane. Total Area: 21 705km². Capricorn District 59.9% of the population is within the 15 to 64 year age group. Municipality falls under the Limpopo province, located on the northern Unemployment rate is at 37.2% with 49.9% of all households that are side of South Africa. It derives its name from the Tropic of Capricorn, female headed. According to Census 2011, half of the population along which it is situated. It is predominantly rural in nature. It of the CDM resides in the Polokwane Municipality, followed by consists of the following five local municipalities: Aganang, Blouberg, Lepelle-Nkumpi, Blouberg and Aganang with 18%, 13% and 10% Lepelle-Nkumpi, Molemole and Polokwane. Limpopo's capital, respectively, while Molemole Local Municipality accounts for 9% Polokwane (previously Pietersburg), lies in the heart of the Capricorn of the population of the district. Although the population of the region. The district has an internal airport, and is linked to Gauteng by district is growing, the rate of growth is declining. -

Post Settlement Challenges for Land Reform Beneficiaries: Three Case Studies from Limpopo Province’ Is My Own Work
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UWC Theses and Dissertations POST SETTLEMENT CHALLENGES FOR LAND REFORM BENEFICIARIES: THREE CASE STUDIES FROM LIMPOPO PROVINCE A mini-thesis submitted in partial fulfilment of the requirements for the degree of Masters Philosophy (Land and Agrarian Studies) Tshililo Justice Manenzhe Programme for Land and Agrarian Studies (PLAAS) Faculty of Economic and Management Sciences University of the Western Cape (UWC) May 2007 DECLARATION I declare that ‘Post Settlement Challenges for Land Reform Beneficiaries: Three case studies from Limpopo Province’ is my own work. All other sources, used or quoted, have been indicated and acknowledged by means of complete references. This thesis has not been submitted for a degree at another university. Tshililo Justice Manenzhe May 2007 Signature ………………………………..…………………… Supervisor: Dr. Edward Lahiff (University of the Western Cape, South Africa) ii ACKNOWLEDGEMENTS Many people have helped me in various ways to complete this thesis, and I thank them. I would like specifically to thank my supervisor, Dr. Edward Lahiff, who patiently read many drafts of this thesis. I am grateful for his support. My gratitude goes to Nkuzi Development Association, my previous employer, for their support and for providing me with financial resources and time off for study purposes. I would like to extend my sincere appreciation to Mr. Marc Wegerif (Ex-director of Nkuzi) for his motivation. I thank the CPA members who welcomed me into their houses, their ploughing fields and their meetings. I am grateful to them because, to the best of their abilities, they shared with me information which in most cases was emotional and sensitive. -

Research Report Restitution and Post-Settlement Support: Three Case Studies from Limpopo Tshililo Manenzhe and Edward Lahiff
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of the Western Cape Research Repository Research Report Restitution and Post-Settlement Support: Three Case Studies from Limpopo Tshililo Manenzhe and Edward Lahiff COMMISSION Department of ON RESTITUTION Land Affairs School of Government, UWC Belgische Technische Coöperatie nv OF LAND RIGHTS Coopération Technique Belge sa Restitution and Post-Settlement Support Three Case studies from Limpopo Tshililo Manenzhe and Edward Lahiff Published by the Programme for Land and Agrarian Studies, School of Government, University of the Western Cape, Private Bag X17, Bellville 7535, Cape Town, South Africa. Tel: +27 21 959 3733. Fax: +27 21 959 3732. E-mail: [email protected] Website: www.plaas.org.za In collaboration with Department of Land Affairs, Commission on Restitution of Land Rights and Belgische Technische Coöperatie (BTC) Programme for Land and Agrarian Studies research report no. 30 ISBN: 978-1-86808-675-7 August 2007 All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without prior permission from the publisher or the authors. Copy editor: Roelien Theron Cover photograph: PLAAS Layout: Designs4development, [email protected] Typeset in Frutiger Printing: RNK Graphics Cartographer: John Hall Research Report Restitution and Post-Settlement Support: Three Case Studies from Limpopo Tshililo Manenzhe and Edward Lahiff This document forms part of a series of reports researched and written by the Sustainable Development Consortium, led by Phuhlisani Solutions, on behalf of the Commission on Restitution of Land Rights and Belgian Technical Cooperation. -

Proposed Upgrade of the Giyani Waste Water Treatment Works, Giyani, Limpopo Province
PROPOSED UPGRADE OF THE GIYANI WASTE WATER TREATMENT WORKS, GIYANI, LIMPOPO PROVINCE Phase 1 – Heritage Impact AssessMent Issue Date - 21 January 2016 Revision No. - 1 Project No. 121HIA PGS Heritage (Pty) Ltd PO Box 32542 Totiusdal 0134, T +27 12 332 5305 F: +27 86 675 8077 Reg No 2003/008940/07 Declaration of Independence The report has been coMpiled by PGS Heritage, an appointed Heritage Specialist for EnvironMental Impact Management Services (Pty) Ltd. The views stipulated in this report are purely objective and no other interests are displayed during the decision Making processes discussed in the Heritage IMpact AssessMent Process . HERITAGE CONSULTANT - PGS Heritage CONTACT PERSON - W Fourie Tel - +27 (0) 12 332 5305 Email - [email protected] SIGNATURE - ______________________________ ACKNOWLEDGEMENT OF RECEIPT CLIENT - EnvironMental IMpact ManageMent Services (Pty) Ltd (EIMS) CONTACT PERSON - Bongani Khupe T: (011) 789-7170 E: [email protected] SIGNATURE - ______________________________ HIA – Project 3: Giyani WWTW ii Date - 21 January 2016 Document Title Proposed upgrade of the Giyani Waste Water TreatMent Works (WWTW), - Giyani, LiMpopo Province. Control Name Signature Designation Author W. Fourie Heritage Specialists/ Principal Investigator Reviewed B Khupe EIMS HIA – Project 3: Giyani WWTW iii EXECUTIVE SUMMARY PGS Heritage (PGS) was appointed by EnvironMental IMpact ManageMent Services (Pty) Ltd (EIMS) to undertake a Heritage IMpact AssessMent (HIA) that forMs part of the Basic Environmental IMpact Report (BAR) for the proposed upgrade of the Giyani Waste Water TreatMent Works (WWTW), Giyani, LiMpopo Province. The following section provides a suMMary of the project locality, scope, heritage resources, envisaged impacts and recoMMendations. 1 SITE NAME Giyani Waste Water TreatMent Works (WWTW), Giyani, LiMpopo Province. -
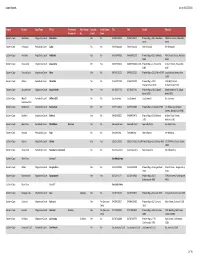
Lowercourts Spreadsheet.Xlsx
Lower Courts As on: 8/25/2010 Region District CourtType Office Previously Also Known Equality Small Claims TEL FAX Postal Physical Known As As Court Court Eastern Cape Aberdeen Magistrate Court Aberdeen Yes No 049 846 0013 049 846 0671 Private Bag x 206, Aberdeen 2A Porter Street, Aberdeen 6270 6270 Eastern Cape Kirkwood Periodical Court Addo No No See Kirkwood See Kirkwood See Kirkwood See Kirkwood Eastern Cape Adelaide Magistrate Court Adelaide Yes No 046 684 0025 046 684 1233 Private Bag x 310, Adelaide 49A Church Street, Adelaide 5760 5760 Eastern Cape Alexandria Magistrate Court Alexandria Yes Yes 046 653 0014 046 653 0164 /1271 Private Bag x 1, Alexandria 2 Court Street, Alexandria 6185 6185 Eastern Cape Victoria East Magistrate Court Alice Yes No 040 653 1121 040 653 2221 Private Bag x 1313, Alice 5700 Long Market Street, Alice 5700 Eastern Cape Albany Periodical Court Alicedale No No 046 622 7303 046 622 5543 Private Bag x 1004, 119A High Street, Grahamstown 6140 Grahamstown 6140 Eastern Cape Aliwal North Magistrate Court Aliwal North Yes Yes 051 633 2173 051 634 2293 Private Bag x 1003, Aliwal‐ Smith Street Nr 15, Aliwal‐ North 9750 North 9750 Eastern Cape Mpofu Periodical Court Balfour [EC] No No See Seymour See Seymour See Seymour See Seymour Stockenström Eastern Cape Barkly East Magistrate Court Barkly East Yes No 045 971 0013 045 971 0585 Private Bag x 1, Barkley 9786 Cnr Molteno & Graham Streets, Barkley‐East 9786 Eastern Cape Bedford Magistrate Court Bedford Yes No 046 865 0020 046 685 0476 Private Bag x 333, Bedford Andreu