Biomarker Testing in Non- Small Cell Lung Cancer (NSCLC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Promoter Janus Kinase 3 Proximal Characterization and Analysis Of
The Journal of Immunology Characterization and Analysis of the Proximal Janus Kinase 3 Promoter1 Martin Aringer,2*† Sigrun R. Hofmann,2* David M. Frucht,* Min Chen,* Michael Centola,* Akio Morinobu,* Roberta Visconti,* Daniel L. Kastner,* Josef S. Smolen,† and John J. O’Shea3* Janus kinase 3 (Jak3) is a nonreceptor tyrosine kinase essential for signaling via cytokine receptors that comprise the common ␥-chain (␥c), i.e., the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. Jak3 is preferentially expressed in hemopoietic cells and is up-regulated upon cell differentiation and activation. Despite the importance of Jak3 in lymphoid development and immune function, the mechanisms that govern its expression have not been defined. To gain insight into this issue, we set out to characterize the Jak3 promoter. The 5-untranslated region of the Jak3 gene is interrupted by a 3515-bp intron. Upstream of this intron and the transcription initiation site, we identified an ϳ1-kb segment that exhibited lymphoid-specific promoter activity and was responsive to TCR signals. Truncation of this fragment revealed that core promoter activity resided in a 267-bp fragment that contains putative Sp-1, AP-1, Ets, Stat, and other binding sites. Mutation of the AP-1 sites significantly diminished, whereas mutation of the Ets sites abolished, the inducibility of the promoter construct. Chromatin immunoprecipitation assays showed that histone acetylation correlates with mRNA expression and that Ets-1/2 binds this region. Thus, transcription factors that bind these sites, especially Ets family members, are likely to be important regulators of Jak3 expression. -

Erb‑B2 Receptor Tyrosine Kinase 2 Is Negatively Regulated by the P53‑Responsive Microrna‑3184‑5P in Cervical Cancer Cells
ONCOLOGY REPORTS 45: 95-106, 2021 Erb‑B2 Receptor Tyrosine Kinase 2 is negatively regulated by the p53‑responsive microRNA‑3184‑5p in cervical cancer cells HONGLI LIU1, YUZHI LI1, JING ZHANG1, NAN WU2, FEI LIU2, LIHUA WANG1, YUAN ZHANG1, JING LIU1, XUAN ZHANG3, SUYANG GUO1 and HONGTAO WANG4 Departments of 1Gynecological Oncology and 2Respiration and Anhui Clinical and Preclinical Key Laboratory of Respiratory Disease, First Affiliated Hospital of Bengbu Medical College; Departments of3 Gynecological Oncology and 4Immunology and Anhui Key Laboratory of Infection and Immunity, Bengbu Medical College, Bengbu, Anhui 233030, P.R. China Received November 30, 2019; Accepted October 2, 2020 DOI: 10.3892/or.2020.7862 Abstract. The oncogenic role of Erb-B2 Receptor Tyrosine Introduction Kinase 2 (ERBB2) has been identified in several types of cancer, but less is known on its function and mechanism of Among women, cervical cancer is ranked 4th in global action in cervical cancer cells. The present study employed cancer-associated deaths (1), with over half a million deaths a multipronged approach to investigate the role of ERBB2 in in 2012 (2). Cervical cancer can be broadly categorized into cervical cancer. ERBB2 and microRNA (miR)-3184-5p expres- squamous cell carcinoma, which constitutes the majority of sion was assessed in patient-derived cervical cancer biopsy cases (70-80%) or adenocarcinoma, which comprises 10-15% tissues, revealing that higher levels of ERBB2 and lower levels of cases (3). Cervical cancer is frequently caused by the of miR-3184-5p were associated with clinicopathological indi- oncovirus human papillomavirus (HPV), mainly by types cators of cervical cancer progression. -

Wt1) – an Effector in Leukemogenesis?
WILMS’ TUMOUR GENE 1 PROTEIN (WT1) – AN EFFECTOR IN LEUKEMOGENESIS? Vidovic, Karina 2010 Link to publication Citation for published version (APA): Vidovic, K. (2010). WILMS’ TUMOUR GENE 1 PROTEIN (WT1) – AN EFFECTOR IN LEUKEMOGENESIS?. Lund University: Faculty of Medicine. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 07. Oct. 2021 Division of Hematology and Transfusion Medicine Lund University, Lund, Sweden WILMS’ TUMOUR GENE 1 PROTEIN (WT1) – AN EFFECTOR IN LEUKEMOGENESIS? Karina Vidovic Thesis 2010 Contact adress Karina Vidovic Division of Hematology and Transfusion Medicine BMC, C14 Klinikgatan 28 SE- 221 84 Lund Sweden Phone +46 46 222 07 30 e-mail: [email protected] ISBN 978-91-86443-99-3 ! Karina Vidovic Printed by Media-Tryck, Lund, Sweden 2 ”The journey of a thousand miles begins with one step” Lao Tzu (Chinese taoist), 600-531 BC 3 4 LIST OF PAPERS This thesis is based on the following papers, referred to in the text by their Roman numerals I. -

JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors
Drugs https://doi.org/10.1007/s40265-020-01261-8 CURRENT OPINION JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors Miguel Nogueira1 · Luis Puig2 · Tiago Torres1,3 © Springer Nature Switzerland AG 2020 Abstract Despite advances in the treatment of psoriasis, there is an unmet need for efective and safe oral treatments. The Janus Kinase– Signal Transducer and Activator of Transcription (JAK–STAT) pathway plays a signifcant role in intracellular signalling of cytokines of numerous cellular processes, important in both normal and pathological states of immune-mediated infamma- tory diseases. Particularly in psoriasis, where the interleukin (IL)-23/IL-17 axis is currently considered the crucial pathogenic pathway, blocking the JAK–STAT pathway with small molecules would be expected to be clinically efective. However, relative non-specifcity and low therapeutic index of the available JAK inhibitors have delayed their integration into the therapeutic armamentarium of psoriasis. Current research appears to be focused on Tyrosine kinase 2 (TYK2), the frst described member of the JAK family. Data from the Phase II trial of BMS-986165—a selective TYK2 inhibitor—in psoriasis have been published and clinical results are encouraging, with a large Phase III programme ongoing. Further, the selective TYK2 inhibitor PF-06826647 is being tested in moderate-to-severe psoriasis in a Phase II clinical trial. Brepocitinib, a potent TYK2/JAK1 inhibitor, is also being evaluated, as both oral and topical treatment. Results of studies with TYK2 inhibitors will be important in assessing the clinical efcacy and safety of these drugs and their place in the therapeutic armamentarium of psoriasis. -

Multiplex Diagnosis of Oncogenic Fusion and MET Exon Skipping by Molecular Counting Using Formalin-Fixed Paraffin Embedded Lung
ORIGINAL ARTICLE Multiplex Diagnosis of Oncogenic Fusion and MET Exon Skipping by Molecular Counting Using Formalin-Fixed Paraffin Embedded Lung Adenocarcinoma Tissues Kuniko Sunami, MD,a,g Koh Furuta, MD, PhD,b Koji Tsuta, MD, PhD,c Shinji Sasada, MD, PhD,d Takehiro Izumo, MD, PhD,d Takashi Nakaoku, MD,a Yoko Shimada, MFSc,a Motonobu Saito, MD, PhD,a Hiroshi Nokihara, MD, PhD,e Shun-ichi Watanabe, MD, PhD,f Yuichiro Ohe, MD, PhD,e,g Takashi Kohno, PhDa,* aDivision of Genome Biology, National Cancer Center Research Institute, Tokyo, Japan bDivision of Clinical Laboratory, National Cancer Center Hospital, Tokyo, Japan cDivision of Pathology, National Cancer Center Research Institute, Tokyo, Japan dDepartment of Endoscopy, Respiratory Endoscopy Division, National Cancer Center Research Institute, Tokyo, Japan eDepartment of Thoracic Oncology, National Cancer Center Research Institute, Tokyo, Japan fDepartment of Thoracic Surgery, National Cancer Center Hospital, Tokyo, Japan gCourse of Advanced Clinical Research of Cancer, Juntendo University Graduate School of Medicine, Tokyo, Japan Received 31 July 2015; revised 23 September 2015; accepted 13 October 2015 ABSTRACT detected oncogenic fusions in bronchial lavage fluid and transbronchial biopsy samples. Introduction: Fusions of the anaplastic lymphoma receptor tyrosine kinase gene (ALK), ret proto-oncogene (RET), ROS Conclusions: The MC assay allows multiplex detection of proto-oncogene 1, receptor tyrosine kinase gene (ROS1), B- oncogenic fusion and exon-skipped transcripts in tumor Raf proto-oncogene, serine/threonine kinase gene (BRAF), samples, including in formalin-fixed paraffin-embedded and neuregulin 1 gene (NRG1) and intronic MMNG HOS samples obtained in the clinic. Transforming gene (MET) mutations are druggable onco- Ó 2015 International Association for the Study of Lung gene alterations in lung adenocarcinoma that cause Cancer. -

(NF1) Defects Are Common in Human Ovarian Serous Carcinomas and Co-Occur with TP53 Mutations
Volume 10 Number 12 December 2008 pp. 1362–1372 1362 www.neoplasia.com Neurofibromin 1 (NF1) Defects Navneet Sangha*, Rong Wu*, Rork Kuick†, Scott Powers‡, David Mu§, Diane Fiander*, Are Common in Human Ovarian Kit Yuen*, Hidetaka Katabuchi¶, Hironori Tashiro¶, Serous Carcinomas and Co-occur Eric R. Fearon*,†,# and Kathleen R. Cho*,†,# 1,2 with TP53 Mutations *Department of Pathology, The University of Michigan Medical School, Ann Arbor, MI 48109, USA; †The Comprehensive Cancer Center, The University of Michigan Medical School, Ann Arbor, MI 48109, USA; ‡Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA; §Department of Pathology, Penn State University College of Medicine, Hershey, PA 17033, USA; ¶Department of Gynecology, Kumamoto University, Kumamoto, 860-8556, Japan; #Department of Internal Medicine, The University of Michigan Medical School, Ann Arbor, MI 48109, USA Abstract Ovarian serous carcinoma (OSC) is the most common and lethal histologic type of ovarian epithelial malignancy. Mutations of TP53 and dysfunction of the Brca1 and/or Brca2 tumor-suppressor proteins have been implicated in the molecular pathogenesis of a large fraction of OSCs, but frequent somatic mutations in other well-established tumor-suppressor genes have not been identified. Using a genome-wide screen of DNA copy number alterations in 36 primary OSCs, we identified two tumors with apparent homozygous deletions of the NF1 gene. Subsequently, 18 ovarian carcinoma–derived cell lines and 41 primary OSCs were evaluated for NF1 alterations. Markedly re- duced or absent expression of Nf1 protein was observed in 6 of the 18 cell lines, and using the protein truncation test and sequencing of cDNA and genomic DNA, NF1 mutations resulting in deletion of exons and/or aberrant splicing of NF1 transcripts were detected in 5 of the 6 cell lines with loss of NF1 expression. -

Promoting Myelin Repair and Return of Function in Multiple Sclerosis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 585 (2011) 3813–3820 journal homepage: www.FEBSLetters.org Review Promoting myelin repair and return of function in multiple sclerosis Jingya Zhang a,b,c, Elisabeth G. Kramer a,b,c, Linnea Asp a,b,c, Dipankar J. Dutta a,b,c, Kristina Navrazhina a,b,c, Trinh Pham a,b,c, John N. Mariani a,b,c, Azeb Tadesse Argaw a,b,c, Carmen V. Melendez-Vasquez d, ⇑ Gareth R. John a,b,c, a Corinne Goldsmith Dickinson Center for MS, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029, USA b Department of Neurology, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029, USA c Friedman Brain Institute, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029, USA d Department of Biological Sciences, Hunter College, 695 Park Avenue, New York, NY 10065, USA article info abstract Article history: Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS. Conduction block in Received 7 July 2011 demyelinated axons underlies early neurological symptoms, but axonal transection and neuronal Revised 8 August 2011 loss are believed to be responsible for more permanent chronic deficits. Several therapies are Accepted 9 August 2011 approved for treatment of relapsing-remitting MS, all of which are immunoregulatory and clinically Available online 18 August 2011 proven to reduce the rate of lesion formation and exacerbation. However, existing approaches are Edited by Richard Williams, Alexander only partially effective in preventing the onset of disability in MS patients, and novel treatments Flügel and Wilhelm Just to protect myelin-producing oligodendrocytes and enhance myelin repair may improve long-term outcomes. -

Angiocrine Endothelium: from Physiology to Cancer Jennifer Pasquier1,2*, Pegah Ghiabi2, Lotf Chouchane3,4,5, Kais Razzouk1, Shahin Rafi3 and Arash Rafi1,2,3
Pasquier et al. J Transl Med (2020) 18:52 https://doi.org/10.1186/s12967-020-02244-9 Journal of Translational Medicine REVIEW Open Access Angiocrine endothelium: from physiology to cancer Jennifer Pasquier1,2*, Pegah Ghiabi2, Lotf Chouchane3,4,5, Kais Razzouk1, Shahin Rafi3 and Arash Rafi1,2,3 Abstract The concept of cancer as a cell-autonomous disease has been challenged by the wealth of knowledge gathered in the past decades on the importance of tumor microenvironment (TM) in cancer progression and metastasis. The sig- nifcance of endothelial cells (ECs) in this scenario was initially attributed to their role in vasculogenesis and angiogen- esis that is critical for tumor initiation and growth. Nevertheless, the identifcation of endothelial-derived angiocrine factors illustrated an alternative non-angiogenic function of ECs contributing to both physiological and pathological tissue development. Gene expression profling studies have demonstrated distinctive expression patterns in tumor- associated endothelial cells that imply a bilateral crosstalk between tumor and its endothelium. Recently, some of the molecular determinants of this reciprocal interaction have been identifed which are considered as potential targets for developing novel anti-angiocrine therapeutic strategies. Keywords: Angiocrine, Endothelium, Cancer, Cancer microenvironment, Angiogenesis Introduction of blood vessels in initiation of tumor growth and stated Metastatic disease accounts for about 90% of patient that in the absence of such angiogenesis, tumors can- mortality. Te difculty in controlling and eradicating not expand their mass or display a metastatic phenotype metastasis might be related to the heterotypic interaction [7]. Based on this theory, many investigators assumed of tumor and its microenvironment [1]. -
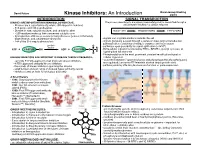
Kinase Inhibitors: an Introduction
David Peters Baran Group Meeting Kinase Inhibitors: An Introduction 2/2/19 INTRODUCTION SIGNAL TRANSDUCTION KINASES ARE IMPORTANT IN HUMAN BIOLOGY/DISEASE: The process describing how a signal (chemical/physical) is transmitted through a - Kinases are a superfamily of proteins (5th largest in humans) cell ultimately resulting in a cellular response - 518 genes and 106 pseudogenes - Diverse in size, subunit structure, and cellular location RECEPTOR TRANSDUCERS EFFECTORS - ~260 residues make up their conserved catalytic core - dysregulation of kinases occurs in many diseases (cancer, inflamatory, degenerative, and autoimmune diseases) - signals can originate inside or outside the cell - 244 of the 518 map to disease loci - signals generally passed through a series of steps (signal transduction pathway) often consisting of multiple enzymes and messengers protein O - pathways open possibility for signal aplification (>1x106) kinase ATP + PROTEIN OH ADP + PROTEIN O P O - Extracellular signals transduced by RTKs, GPCR’s, guanlyl cyclases, or ligand-gated Ion channels O - Phosphorylation is the most prominent covalent modification/signal in KINASES INHIBITORS ARE IMPORTANT IN DISEASE THERAPY/RESEARCH: cellular regulation - currently 51 FDA approved small molecule kinase inhibitors - “converter enzymes” (protein kinases and phosphoprotein phosphorlyases) - 4 FDA approved antibody kinase inhibitors are regulated; conserve ATP/maintain desired target protein state - thousands of known inhibitors spanning the kinome - pathway ends by affecting biomolecule -

The Role of Signaling Pathways in the Development and Treatment of Hepatocellular Carcinoma
Oncogene (2010) 29, 4989–5005 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc REVIEW The role of signaling pathways in the development and treatment of hepatocellular carcinoma S Whittaker1,2, R Marais3 and AX Zhu4 1Dana-Farber Cancer Institute, Boston, MA, USA; 2The Broad Institute, Cambridge, MA, USA; 3Institute of Cancer Research, London, UK and 4Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA Hepatocellular carcinoma (HCC) is a highly prevalent, malignancy in adults (Pons-Renedo and Llovet, 2003). treatment-resistant malignancy with a multifaceted mole- For the vast majority of patients, HCC is a late cular pathogenesis. Current evidence indicates that during complication of chronic liver disease, and as such, is hepatocarcinogenesis, two main pathogenic mechanisms often associated with cirrhosis. The main risk factors for prevail: (1) cirrhosis associated with hepatic regeneration the development of HCC include infection with hepatitis after tissue damage caused by hepatitis infection, toxins B virus (HBV) or hepatitis C virus (HCV). Hepatitis (for example, alcohol or aflatoxin) or metabolic influ- infection is believed to be the main etiologic factor in ences, and (2) mutations occurring in single or multiple 480% of cases (Anzola, 2004). Other risk factors oncogenes or tumor suppressor genes. Both mechanisms include excessive alcohol consumption, nonalcoholic have been linked with alterations in several important steatohepatitis, autoimmune hepatitis, primary biliary cellular signaling pathways. These pathways are of cirrhosis, exposure to environmental carcinogens (parti- interest from a therapeutic perspective, because targeting cularly aflatoxin B) and the presence of various genetic them may help to reverse, delay or prevent tumorigenesis. -

Epigenetic Gene Regulation by Janus Kinase 1 in Diffuse Large B-Cell Lymphoma
Epigenetic gene regulation by Janus kinase 1 in diffuse large B-cell lymphoma Lixin Ruia,b,c,1,2, Amanda C. Drennanb,c,1, Michele Ceribellia,1, Fen Zhub,c, George W. Wrightd, Da Wei Huanga, Wenming Xiaoe, Yangguang Lib,c, Kreg M. Grindleb,c,LiLub,c, Daniel J. Hodsona, Arthur L. Shaffera, Hong Zhaoa, Weihong Xua, Yandan Yanga, and Louis M. Staudta,2 aLymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, MD 20892; bDepartment of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, WI 53705; cCarbone Cancer Center, School of Medicine and Public Health, University of Wisconsin, Madison, WI 53705; dBiometric Research Branch, DCTD, National Cancer Institute, NIH, Bethesda, MD 20892; and eDivision of Bioinformatics and Biostatistics, National Center for Toxicological Research/Food and Drug Administration, Jefferson, AR 72079 Contributed by Louis M. Staudt, September 29, 2016 (sent for review July 22, 2016; reviewed by Anthony R. Green and Ross L. Levine) Janus kinases (JAKs) classically signal by activating STAT transcription promote STAT dimerization, nuclear translocation, and binding factors but can also regulate gene expression by epigenetically to cis-regulatory elements to regulate transcription (15, 17). This phosphorylating histone H3 on tyrosine 41 (H3Y41-P). In diffuse large canonical JAK/STAT pathway is deregulated in several hemato- B-cell lymphomas (DLBCLs), JAK signaling is a feature of the activated logic malignancies (16). In DLBCL, STAT3 is activated in the B-cell (ABC) subtype and is triggered by autocrine production of IL-6 ABC subtype and regulates gene expression to promote the sur- and IL-10. -

Targeting the Function of the HER2 Oncogene in Human Cancer Therapeutics
Oncogene (2007) 26, 6577–6592 & 2007 Nature Publishing Group All rights reserved 0950-9232/07 $30.00 www.nature.com/onc REVIEW Targeting the function of the HER2 oncogene in human cancer therapeutics MM Moasser Department of Medicine, Comprehensive Cancer Center, University of California, San Francisco, CA, USA The year 2007 marks exactly two decades since human HER3 (erbB3) and HER4 (erbB4). The importance of epidermal growth factor receptor-2 (HER2) was func- HER2 in cancer was realized in the early 1980s when a tionally implicated in the pathogenesis of human breast mutationally activated form of its rodent homolog neu cancer (Slamon et al., 1987). This finding established the was identified in a search for oncogenes in a carcinogen- HER2 oncogene hypothesis for the development of some induced rat tumorigenesis model(Shih et al., 1981). Its human cancers. An abundance of experimental evidence human homologue, HER2 was simultaneously cloned compiled over the past two decades now solidly supports and found to be amplified in a breast cancer cell line the HER2 oncogene hypothesis. A direct consequence (King et al., 1985). The relevance of HER2 to human of this hypothesis was the promise that inhibitors of cancer was established when it was discovered that oncogenic HER2 would be highly effective treatments for approximately 25–30% of breast cancers have amplifi- HER2-driven cancers. This treatment hypothesis has led cation and overexpression of HER2 and these cancers to the development and widespread use of anti-HER2 have worse biologic behavior and prognosis (Slamon antibodies (trastuzumab) in clinical management resulting et al., 1989).