Benguela Current Forage Fish Workshop Ecosystem Thresholds As
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fronts in the World Ocean's Large Marine Ecosystems. ICES CM 2007
- 1 - This paper can be freely cited without prior reference to the authors International Council ICES CM 2007/D:21 for the Exploration Theme Session D: Comparative Marine Ecosystem of the Sea (ICES) Structure and Function: Descriptors and Characteristics Fronts in the World Ocean’s Large Marine Ecosystems Igor M. Belkin and Peter C. Cornillon Abstract. Oceanic fronts shape marine ecosystems; therefore front mapping and characterization is one of the most important aspects of physical oceanography. Here we report on the first effort to map and describe all major fronts in the World Ocean’s Large Marine Ecosystems (LMEs). Apart from a geographical review, these fronts are classified according to their origin and physical mechanisms that maintain them. This first-ever zero-order pattern of the LME fronts is based on a unique global frontal data base assembled at the University of Rhode Island. Thermal fronts were automatically derived from 12 years (1985-1996) of twice-daily satellite 9-km resolution global AVHRR SST fields with the Cayula-Cornillon front detection algorithm. These frontal maps serve as guidance in using hydrographic data to explore subsurface thermohaline fronts, whose surface thermal signatures have been mapped from space. Our most recent study of chlorophyll fronts in the Northwest Atlantic from high-resolution 1-km data (Belkin and O’Reilly, 2007) revealed a close spatial association between chlorophyll fronts and SST fronts, suggesting causative links between these two types of fronts. Keywords: Fronts; Large Marine Ecosystems; World Ocean; sea surface temperature. Igor M. Belkin: Graduate School of Oceanography, University of Rhode Island, 215 South Ferry Road, Narragansett, Rhode Island 02882, USA [tel.: +1 401 874 6533, fax: +1 874 6728, email: [email protected]]. -
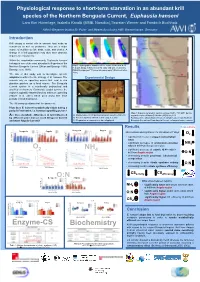
Physiological Response to Short-Term Starvation in an Abundant Krill
Physiological response to short-term starvation in an abundant krill species of the Northern Benguela Current, Euphausia hanseni Lara Kim Hünerlage, Isabella Kandjii (MME, Namibia),Thorsten Werner and Friedrich Buchholz Alfred-Wegener-Institut für Polar- und Meeresforschung AWI, Bremerhaven, Germany Introduction Krill occupy a central role in oceanic food webs as consumers as well as producers. They are a major source of nutrition to fish, birds, seals, and whales. A change in a krill population may thus have dramatic impacts on ecosystems. Within the zooplankton community, Euphausia hanseni belongs to one of the most abundant krill species of the Northern Benguela Current (Olivar and Barange 1990; Map 1: Hydrographic situation off the coast of Namibia at 20 m depth. Image is based on CTD data and was created by Barange et al. 1991). GENUS-subproject “Physical Oceanography” (Mohrholz et al. 2011). The aim of this study was to investigate specific adaptations within the life strategy of E. hanseni. The Experimental Design animals rely on upwelling pulses that lead to rich plankton patches as a food source. The Benguela Current system is a nutritionally poly-pulsed and stratified environment. During late austral summer, the region is typically characterized by minimum upwelling A (Hagen et al. 2001) which goes along with short periods of food deprivation. The following questions shall be answered: How does E. hanseni metabolically adjust during a period of starvation, i.e. between upwelling pulses? C B Map 2: Stations sampled in austral summer 30.01- 7.08.2011 during Are there metabolic differences in krill influenced A) Maintenance of krill during starvation experiment (n=48) research cruise of Maria S. -

St Helena Bay
The variability of retention in St Helena Bay Anathi Manyakanyaka MNYANA002 Supervisors: Dr Jenifer Jackson-Veitch (SAEON) and A/Prof Mathieu Rouault (UCT) A minor dissertation submitted in partial fulfilment of the requirements for the degree of Master of Science in Applied Ocean Sciences of the University of Cape Town University of Cape Town Department of Oceanography Faculty of Science Submitted February 2020 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Declaration I know that plagiarism is wrong. Plagiarism is to use another’s work and pretend that it is one’s own. I have used the Harvard Style referencing convention for citation and referencing. Each contribution to, and quotation in, this thesis from the work(s) of other people has been attributed and has been cited and referenced. This thesis is my own work. I have not allowed, and will not allow, anyone to copy my work with the intention of passing it off as his or her own work. Signature ______________________________ Date 06 February 2020 _________________ 1 | Page Abstract The circulation in St Helena Bay and the variability of the retention of the Bay are investigated using seasonal climatologies of the Regional Ocean Modelling System (ROMS). While retention has been studied biologically, the seasonality of the hydrodynamics contributing to the retention have received less attention. -

Characteristics of Intermediate Water Flow in the Benguela Current As
Deep-Sea Research II 50 (2003) 87–118 Characteristics of intermediate water flow in the Benguela current as measured with RAFOS floats P.L. Richardsona,*, S.L. Garzolib a Department of Physical Oceanography, Woods Hole Oceanographic Institution, 360 Woods Hole Road, Woods Hole, MA 02543, 3 Water Street, P.O. Box 721, USA b Atlantic Oceanographic and Meteorological Laboratory, NOAA, 4301 Rickenbacker Causeway, Miami, FL 33149, USA Received 28 September 2001; accepted 26 July 2002 Abstract Seven floats (not launched in rings) crossed over the mid-Atlantic Ridge in the Benguela extension with a mean westward velocity of around 2 cm=s between 22S and 35S. Two Agulhas rings crossed over the mid-Atlantic Ridge with a mean velocity of 5:7cm=s toward 2851: This implies they translated at around 3:8cm=s through the background velocity field near 750 m: The boundaries of the Benguela Current extension were clearly defined from the observations. At 750 m the Benguela extension was bounded on the south by 35S and the north by an eastward current located between 18S and 21S. Other recent float measurements suggest that this eastward current originates near the Trindade Ridge close to the western boundary and extends across most of the South Atlantic, limiting the Benguela extension from flowing north of around 20S. The westward transport of the Benguela extension was estimated to be 15 Sv by integrating the mean westward velocities from 22S to 35S and multiplying by the 500 m estimated thickness of intermediate water. Roughly 1.5 Sv of this are transported by the B3 Agulhas rings that cross the mid-Atlantic Ridge each year (as observed with altimetry). -

Lecture 4: OCEANS (Outline)
LectureLecture 44 :: OCEANSOCEANS (Outline)(Outline) Basic Structures and Dynamics Ekman transport Geostrophic currents Surface Ocean Circulation Subtropicl gyre Boundary current Deep Ocean Circulation Thermohaline conveyor belt ESS200A Prof. Jin -Yi Yu BasicBasic OceanOcean StructuresStructures Warm up by sunlight! Upper Ocean (~100 m) Shallow, warm upper layer where light is abundant and where most marine life can be found. Deep Ocean Cold, dark, deep ocean where plenty supplies of nutrients and carbon exist. ESS200A No sunlight! Prof. Jin -Yi Yu BasicBasic OceanOcean CurrentCurrent SystemsSystems Upper Ocean surface circulation Deep Ocean deep ocean circulation ESS200A (from “Is The Temperature Rising?”) Prof. Jin -Yi Yu TheThe StateState ofof OceansOceans Temperature warm on the upper ocean, cold in the deeper ocean. Salinity variations determined by evaporation, precipitation, sea-ice formation and melt, and river runoff. Density small in the upper ocean, large in the deeper ocean. ESS200A Prof. Jin -Yi Yu PotentialPotential TemperatureTemperature Potential temperature is very close to temperature in the ocean. The average temperature of the world ocean is about 3.6°C. ESS200A (from Global Physical Climatology ) Prof. Jin -Yi Yu SalinitySalinity E < P Sea-ice formation and melting E > P Salinity is the mass of dissolved salts in a kilogram of seawater. Unit: ‰ (part per thousand; per mil). The average salinity of the world ocean is 34.7‰. Four major factors that affect salinity: evaporation, precipitation, inflow of river water, and sea-ice formation and melting. (from Global Physical Climatology ) ESS200A Prof. Jin -Yi Yu Low density due to absorption of solar energy near the surface. DensityDensity Seawater is almost incompressible, so the density of seawater is always very close to 1000 kg/m 3. -

Atlantic Ocean Equatorial Currents
188 ATLANTIC OCEAN EQUATORIAL CURRENTS ATLANTIC OCEAN EQUATORIAL CURRENTS S. G. Philander, Princeton University, Princeton, Centered on the equator, and below the westward NJ, USA surface Sow, is an intense eastward jet known as the Equatorial Undercurrent which amounts to a Copyright ^ 2001 Academic Press narrow ribbon that precisely marks the location of doi:10.1006/rwos.2001.0361 the equator. The undercurrent attains speeds on the order of 1 m s\1 has a half-width of approximately Introduction 100 km; its core, in the thermocline, is at a depth of approximately 100 m in the west, and shoals to- The circulations of the tropical Atlantic and PaciRc wards the east. The current exists because the west- Oceans have much in common because similar trade ward trade winds, in addition to driving divergent winds, with similar seasonal Suctuations, prevail westward surface Sow (upwelling is most intense at over both oceans. The salient features of these circu- the equator), also maintain an eastward pressure lations are alternating bands of eastward- and west- force by piling up the warm surface waters in the ward-Sowing currents in the surface layers (see western side of the ocean basin. That pressure force Figure 1). Fluctuations of the currents in the two is associated with equatorward Sow in the thermo- oceans have similarities not only on seasonal but cline because of the Coriolis force. At the equator, even on interannual timescales; the Atlantic has where the Coriolis force vanishes, the pressure force a phenomenon that is the counterpart of El Ninoin is the source of momentum for the eastward Equa- the PaciRc. -

Upper-Level Circulation in the South Atlantic Ocean
Prog. Oceanog. Vol. 26, pp. 1-73, 1991. 0079 - 6611/91 $0.00 + .50 Printed in Great Britain. All fights reserved. © 1991 Pergamon Press pie Upper-level circulation in the South Atlantic Ocean RAY G. P~-rwtSON and LOTHAR Sa~AMMA lnstitut fiir Meereskunde an der Universitiit Kiel, Diisternbrooker Weg 20, 2300 Kiel 1, F.R.G. Abstract - In this paper we present a literature survey of the South Atlantic's climate and its oceanic upper-layer circulation and meridional beat transport. The opening section deals with climate and is focused upon those elements having greatest oceanic relevance, i.e., distributions of atmospheric sea level pressure, the wind fields they produce, and the net surface energy fluxes. The various geostrophic currents comprising the upper-level general circulation are then reviewed in a manner organized around the subtropical gyre, beginning off southern Africa with the Agulhas Current Retroflection and then progressing to the Benguela Current, the equatorial current system and circulation in the Angola Basin, the large-scale variability and interannual warmings at low latitudes, the Brazil Current, the South Atlantic Cmrent, and finally to the Antarctic Circumpolar Current system in which the Falkland (Malvinas) Current is included. A summary of estimates of the meridional heat transport at various latitudes in the South Atlantic ends the survey. CONTENTS 1. Introduction 2 2. Climatic Elements 2 3. Subtropical and Equatorial Circulation 11 3.1. Agulhas Current Retroflection 11 3.2. Benguela Cmrent 16 3.3. Equatorial Cttrrents 18 3.3.1. Components of the system 18 3.3.2. Angola Basin circulation 26 3.3.3. -

Benguela Current Large Marine Ecosystem (LME)
OPTIONAL ANNEXES Identifiers: Project Number: PIMS: 0096 UNDP: RAF00G31 Project Name: Implementation of the Strategic Action Program (SAP) Toward Achievement of the Integrated Management of the Benguela Current Large Marine Ecosystem (LME) Annex 5: Transboundary Diagnostic Analysis Annex 6: Strategic Action Programme Annex 7: Summary of the Functions and Responsibilities of the Interim Benguela Current Commission (IBCC) Annex 8: Thematic Reports Prepared During the PDF-B Project Phase Annex 9: Stakeholders Involvement Description and List of the Stakeholders Participants 2 ANNEX H BENGUELA CURRENT LARGE MARINE ECOSYSTEM PROGRAMME (BCLME) A regional commitment to the sustainable integrated management of the Benguela Current Large Marine Ecosystem by Angola, Namibia and South Africa TRANSBOUNDARY DIAGNOSTIC ANALYSIS (TDA) UNDP WINDHOEK, OCTOBER 1999 3 TABLE OF CONTENTS Page Background and Introduction 1 * The Benguela: a unique environment 1 * Fragmented management: a legacy of the 2 colonial and political past * The need for international action 3 * The success story of BENEFIT 4 * The emerging BCLME Programme 6 * What has been achieved 6 * Towards a sustainable future: the next steps 8 Users Guide to the Transboundary Diagnostic Analysis 11 * Definitions and TDA objective 11 * Design of the TDA 11 (a) Level One: Synthesis (b) Level Two: Specifics * More information 12 BCLME Transboundary Diagnostic Analysis 13 * Geographic scope and ecosystem boundaries 13 * Level One: Synthesis 14 * Synthesis Matrix 16 * Level Two: Overview 17 * Analysis -

Krill in the Arctic and the Atlantic – Climatic Variability and Adaptive Capacity –
Krill in the Arctic and the Atlantic – Climatic Variability and Adaptive Capacity – Dissertation with the Aim of Achieving a Doctoral Degree in Natural Science – Dr. rer. nat. – at the Faculty of Mathematics, Informatics and Natural Sciences Department of Biology of the University of Hamburg submitted by Lara Kim Hünerlage M.Sc. Marine Biology B.Sc. Environmental Science Hamburg 2015 This cumulative dissertation corresponds to the exam copy (submitted November 11th, 2014). The detailed content of the single publications may have changed during the review processes. Please contact the author for citation purposes. Day of oral defence: 20th of February, 2015 The following evaluators recommend the acceptance of the dissertation: 1. Evaluator Prof. Dr. Friedrich Buchholz Institut für Hydrobiologie und Fischereiwissenschaft, Fakultät für Mathematik, Informatik und Naturwissenschaften, Universität Hamburg; Alfred-Wegner-Institut Helmholtz Zentrum für Polar- und Meeresforschung, Funktionelle Ökologie, Bremerhaven 2. Evaluator Prof. Dr. Myron Peck Institut für Hydrobiologie und Fischereiwissenschaft, Fakultät für Mathematik, Informatik und Naturwissenschaften, Universität Hamburg 3. Evaluator Prof. Dr. Ulrich Bathmann Leibniz-Institut für Ostseeforschung Warnemünde; Interdisziplinäre Fakultät für Maritime Systeme, Universität Rostock IN MEMORY OF MY FATHER, GERD HÜNERLAGE DEDICATED TO MY FAMILY PREFACE This cumulative dissertation summarizes the research findings of my PhD project which was conducted from September 2011 to October 2014. Primarily, -

South Atlantic
IOC-UNESCO TS129 What are Marine Ecological Time Series telling us about the ocean? A status report [ Individual Chapter (PDF) download ] The full report (all chapters and Annex) is available online at: http://igmets.net/report Chapter 01: New light for ship-based time series (Introduction) Chapter 02: Methods & Visualizations Chapter 03: Arctic Ocean Chapter 04: North Atlantic Chapter 05: South Atlantic Chapter 06: Southern Ocean Chapter 07: Indian Ocean Chapter 08: South Pacific Chapter 09: North Pacific Chapter 10: Global Overview Annex: Directory of Time-series Programmes This page intentionally left blank to preserve pagination in double-sided (booklet) printing 2 Chapter 5 South Atlantic Ocean 5 South Atlantic Ocean Frank E. Muller-Karger, Alberto Piola, Hans M. Verheye, Todd D. O’Brien, and Laura Lorenzoni Figure 5.1. Map of IGMETS-participating South Atlantic time series on a background of a 10-year time-window (2003–2012) sea surface temperature trends (see also Figure 5.3). At the time of this report, the South Atlantic collection consisted of 13 time series (coloured symbols of any type), of which two were from estuarine areas (yellow stars). Dashed lines indicate boundaries between IGMETS regions. Uncoloured (gray) symbols indicate time series being addressed in a different regional chapter (e.g. Southern Ocean, South Pacific, North Atlantic). See Table 5.3 for a listing of this region’s participating sites. Additional information on the sites in this study is presented in the Annex. Participating time-series investigators Carla F. Berghoff, Mario O. Carignan, Fabienne Cazassus, Georgina Cepeda, Paulo Cesar Abreu, Rudi Cloete, Maria Constanza Hozbor, Daniel Cucchi Colleoni, Valeria Guinder, Richard Horaeb, Jenny Huggett, Anja Kreiner, Ezequiel Leonarduzzi, Vivian Lutz, Jorge Marcovecchio, Graciela N. -

Benguela Water Masses Paper
A demonstration of the hydrographic partition of the Benguela upwelling ecosystem at 26◦400S Christopher M. Duncombe Rae∗ Submitted to Afr. J. mar. Sci. 10th May, 2004 Revised 3rd September, 2004 (Version: 5.1, September 13, 2004) ∗Marine & Coastal Management, Department of Environmental Affairs and Tourism, Private Bag X2, Roggebaai 8012, South Africa. E-mail: [email protected] 1 Duncombe Rae: Hydrographic partition of the Benguela Abstract Continuous CTD data from a series of recent cruises show that the distribution of the water mass characteristics in the central Benguela region from the Orange River mouth (28◦380S) to Walvis Bay (22◦570S) is discontinuous in the central and intermediate waters at about the latitude of Luderitz¨ (26◦400S). The central and intermediate water masses at the shelf edge and shelf break north of the Luderitz¨ upwelling cell have a high salinity relative to the potential temperature compared to similar waters south of the upwelling cell. It is shown that the feed waters for the wind-induced upwelling on the shelf to the north and south of the Luderitz¨ discontinuity are different in character and source. The distribution of the water masses shows that the shelf-edge poleward undercurrent provides low- oxygen water from different regions in the Atlantic Ocean to be upwelled onto the shelf. North of the Luderitz¨ upwelling cell, the central and intermediate waters come from the oxygen-depleted Angola Basin, while south of the discontinuity those waters are from the interior of the adjacent Cape Basin, which is less oxygen deficient. This has implications for the dispersion of low oxygen water and the triggering of anoxic events, and consequences for the biota on the shelf, including commercially important fish species. -

Ocean Climate of the South East Atlantic Observed from Satellite Data and Wind Models N.J
Progress in Oceanography 59 (2003) 181–221 www.elsevier.com/locate/pocean Ocean climate of the South East Atlantic observed from satellite data and wind models N.J. Hardman-Mountford a,∗, A.J. Richardson b, 1, J.J. Agenbag c, E. Hagen d, L. Nykjaer e, F.A. Shillington b, C. Villacastin e a Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth, Devon PL1 2PB, UK b Oceanography Department, University of Cape Town, Rondebosch 7701, Cape Town, South Africa c Marine and Coastal Management, Private Bag X2, Rogge Bay, 8012 Cape Town, South Africa d Insitute for Baltic Sea Research Warnemuende, Seestrasse 15, 19119 Warnemuende, Germany e Institute for Environment and Sustainability, Joint Research Centre, I-21020 Ispra, Va, Italy Revised 8 September 2003; accepted 14 October 2003 Abstract The near-coastal South East Atlantic Ocean off Africa is a unique and highly dynamic environment, comprising the cool Benguela Current, warm Angola Current and warm Agulhas Current. Strong coastal upwelling and the Congo River strongly influence primary production. Much of the present knowledge of the South East Atlantic has been derived from ship-borne measurements and in situ sensors, which cannot generally provide extensive spatial and tem- poral coverage. Similarly, previous satellite studies of the region have often focused on small spatial areas and limited time periods. This paper provides an improved understanding of seasonal and interannual variability in ocean dynamics along the South East Atlantic coast of Africa using time series of satellite and model derived data products. Eighteen years of satellite sea surface temperature data are complimented by 7 years of sea level data.