Although Most of the Phytoplankton of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fronts in the World Ocean's Large Marine Ecosystems. ICES CM 2007
- 1 - This paper can be freely cited without prior reference to the authors International Council ICES CM 2007/D:21 for the Exploration Theme Session D: Comparative Marine Ecosystem of the Sea (ICES) Structure and Function: Descriptors and Characteristics Fronts in the World Ocean’s Large Marine Ecosystems Igor M. Belkin and Peter C. Cornillon Abstract. Oceanic fronts shape marine ecosystems; therefore front mapping and characterization is one of the most important aspects of physical oceanography. Here we report on the first effort to map and describe all major fronts in the World Ocean’s Large Marine Ecosystems (LMEs). Apart from a geographical review, these fronts are classified according to their origin and physical mechanisms that maintain them. This first-ever zero-order pattern of the LME fronts is based on a unique global frontal data base assembled at the University of Rhode Island. Thermal fronts were automatically derived from 12 years (1985-1996) of twice-daily satellite 9-km resolution global AVHRR SST fields with the Cayula-Cornillon front detection algorithm. These frontal maps serve as guidance in using hydrographic data to explore subsurface thermohaline fronts, whose surface thermal signatures have been mapped from space. Our most recent study of chlorophyll fronts in the Northwest Atlantic from high-resolution 1-km data (Belkin and O’Reilly, 2007) revealed a close spatial association between chlorophyll fronts and SST fronts, suggesting causative links between these two types of fronts. Keywords: Fronts; Large Marine Ecosystems; World Ocean; sea surface temperature. Igor M. Belkin: Graduate School of Oceanography, University of Rhode Island, 215 South Ferry Road, Narragansett, Rhode Island 02882, USA [tel.: +1 401 874 6533, fax: +1 874 6728, email: [email protected]]. -

Assessing the Potential for Range Expansion of the Red Tide Algae Karenia Brevis
Nova Southeastern University NSUWorks All HCAS Student Capstones, Theses, and Dissertations HCAS Student Theses and Dissertations 8-7-2020 Assessing the Potential for Range Expansion of the Red Tide Algae Karenia brevis Edward W. Young Follow this and additional works at: https://nsuworks.nova.edu/hcas_etd_all Part of the Marine Biology Commons Share Feedback About This Item NSUWorks Citation Edward W. Young. 2020. Assessing the Potential for Range Expansion of the Red Tide Algae Karenia brevis. Capstone. Nova Southeastern University. Retrieved from NSUWorks, . (13) https://nsuworks.nova.edu/hcas_etd_all/13. This Capstone is brought to you by the HCAS Student Theses and Dissertations at NSUWorks. It has been accepted for inclusion in All HCAS Student Capstones, Theses, and Dissertations by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Capstone of Edward W. Young Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science Marine Science Nova Southeastern University Halmos College of Arts and Sciences August 2020 Approved: Capstone Committee Major Professor: D. Abigail Renegar, Ph.D. Committee Member: Robert Smith, Ph.D. This capstone is available at NSUWorks: https://nsuworks.nova.edu/hcas_etd_all/13 Nova Southeastern Univeristy Halmos College of Arts and Sciences Assessing the Potential for Range Expansion of the Red Tide Algae Karenia brevis By Edward William Young Submitted to the Faculty of Halmos College of Arts and Sciences in partial fulfillment of the requirements for the degree of Masters of Science with a specialty in: Marine Biology Nova Southeastern University September 8th, 2020 1 Table of Contents 1. -

Wednesday Update Marine Lab Monitoring Red Tide Around Islands
Wednesday Update January 13, 2021 Welcome to the first 2021 edition of the Wednesday Update! We'll email the next issue on Jan. 27. By highlighting SCCF's mission to protect and care for Southwest Florida's coastal ecosystems, our updates connect you to nature. Thanks to Frances Tutt for this photo of American white pelicans (Pelecanus erythrorhynchos) feeding. DO YOU HAVE WILDLIFE PHOTOS TO SHARE? Please send your photos to [email protected] to be featured in an upcoming issue. Marine Lab Monitoring Red Tide Around Islands Today's daily sampling map from the Florida Fish and Wildlife Conservation Commission (FWC), pictured here, shows that a patchy bloom of the red tide organism, Karenia brevis, persists in Southwest Florida based on sampling conducted over the past eight days. This afternoon's mid-week update reported that background to high concentrations of K. brevis were detected in 42 samples over the past week. Medium bloom concentrations (>100,000 cells/liter) were observed in 32 samples collected from Lee and Collier counties, according to the FWC. As indicated by the dots on this map, background to high concentrations were recorded in Lee County in 24 samples, and medium to high concentrations in and offshore of Collier County were observed in 17 samples. Daily samples collected by SCCF's Marine Lab and Sanibel Sea School at local beaches and back bay waters have ranged from high concentrations (>1 million K. brevis cells/liter) to low (>10,000 cells/liter). "Today we counted low levels mid-island on Sanibel and a high level at the Lighthouse," said SCCF Research Scientist Rick Bartleson. -

Subsurface Dinoflagellate Populations, Frontal Blooms and the Formation of Red Tide in the Southern Benguela Upwelling System
MARINE ECOLOGY PROGRESS SERIES Vol. 172: 253-264. 1998 Published October 22 Mar Ecol Prog Ser Subsurface dinoflagellate populations, frontal blooms and the formation of red tide in the southern Benguela upwelling system G. C. Pitcher*,A. J. Boyd, D. A. Horstman, B. A. Mitchell-Innes Sea Fisheries Research Institute, Private Bag X2, Rogge Bay 8012, Cape Town. South Africa ABSTRACT- The West Coast of South Africa is often subjected to problems associated with red tides which are usually attributed to blooms of migratory dinoflagellates. This study investigates the cou- pling between the physical environment and the biological behaviour and physiological adaptation of dinoflagellates in an attempt to understand bloom development, maintenance and decline. Widespread and persistent subsurface dinoflagellate populations domlnate the stratified waters of the southern Benguela during the latter part of the upwelling season. Chlorophyll concentrations as high as 50 mg m-3 are associated with the the]-mocline at approximately 20 m depth but photosynthesis in this region is restricted by low light. The subsurface population is brought to the surface in the region of the upwelling front. Here increased light levels are responsible for enhanced production, in some instances exceeding 80 mgC rn.' h ', and resulting in dense dinoflagellate concentrations in and around the uplifted thermocline. Under particular wind and current conditions these frontal bloon~sare trans- ported and accumulated inshore and red tides are formed. KEY WORDS: Dinoflagellates Subsurface populations . Frontal bloolns Red tide - Upwelling systems INTRODUCTION Pitcher 1996), or from physical damage, such as the clogging of fish gills (Grindley & Nel 1968, Brown et al. -

A Study on Red Tide Risk and Basic Understanding of Fishermen and Residents in Bandar Abbas, Hormozgan Province, Iran (Persian Gulf)
Iranian Journal of Fisheries Sciences 19(1) 471-487 2020 DOI: 10.22092/ijfs.2019.119690. A study on red tide risk and basic understanding of fishermen and residents in Bandar Abbas, Hormozgan Province, Iran (Persian Gulf) Mirza Esmaeili F.1; Mortazavi M.S.2*; Arjmandi R.1; Lahijanian A.1 Received: August 2017 Accepted: January 2018 Abstract Harmful algal bloom can be regarded as a persistence environmental problem in the Persian Gulf. This region has experienced many problematic human and social issues, economic damages, and environmental problems caused by red tides. However, no coherent study has been devoted to shed light on perceptions of red tide risks in the Persian Gulf. In response to the mentioned gap, the present study aimed to investigate residents and fishermen perceptions of red tide risks in Bandar Abbas. To meet the mentioned objective, a total of 247 and 145 subjects filled out structured questionnaires in two coastal parks and Bandar Abbas Fishermen, respectively. The obtained results indicated that the demographic factors along with experience and human health issues of red tides affected subjects’ risk perception. This study also revealed that fishermens and residents intensify the risk of red tides, such as seafood consumption, occurrence of red tides and their progress towards coast. Negative media coverage, limited information, and lack of any support of fishermen by government are some of the factors affecting individuals’ reaction and concerns towards red tide. Downloaded from jifro.ir at 0:22 +0330 on Monday September -
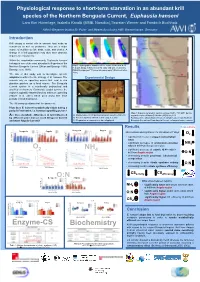
Physiological Response to Short-Term Starvation in an Abundant Krill
Physiological response to short-term starvation in an abundant krill species of the Northern Benguela Current, Euphausia hanseni Lara Kim Hünerlage, Isabella Kandjii (MME, Namibia),Thorsten Werner and Friedrich Buchholz Alfred-Wegener-Institut für Polar- und Meeresforschung AWI, Bremerhaven, Germany Introduction Krill occupy a central role in oceanic food webs as consumers as well as producers. They are a major source of nutrition to fish, birds, seals, and whales. A change in a krill population may thus have dramatic impacts on ecosystems. Within the zooplankton community, Euphausia hanseni belongs to one of the most abundant krill species of the Northern Benguela Current (Olivar and Barange 1990; Map 1: Hydrographic situation off the coast of Namibia at 20 m depth. Image is based on CTD data and was created by Barange et al. 1991). GENUS-subproject “Physical Oceanography” (Mohrholz et al. 2011). The aim of this study was to investigate specific adaptations within the life strategy of E. hanseni. The Experimental Design animals rely on upwelling pulses that lead to rich plankton patches as a food source. The Benguela Current system is a nutritionally poly-pulsed and stratified environment. During late austral summer, the region is typically characterized by minimum upwelling A (Hagen et al. 2001) which goes along with short periods of food deprivation. The following questions shall be answered: How does E. hanseni metabolically adjust during a period of starvation, i.e. between upwelling pulses? C B Map 2: Stations sampled in austral summer 30.01- 7.08.2011 during Are there metabolic differences in krill influenced A) Maintenance of krill during starvation experiment (n=48) research cruise of Maria S. -

St Helena Bay
The variability of retention in St Helena Bay Anathi Manyakanyaka MNYANA002 Supervisors: Dr Jenifer Jackson-Veitch (SAEON) and A/Prof Mathieu Rouault (UCT) A minor dissertation submitted in partial fulfilment of the requirements for the degree of Master of Science in Applied Ocean Sciences of the University of Cape Town University of Cape Town Department of Oceanography Faculty of Science Submitted February 2020 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Declaration I know that plagiarism is wrong. Plagiarism is to use another’s work and pretend that it is one’s own. I have used the Harvard Style referencing convention for citation and referencing. Each contribution to, and quotation in, this thesis from the work(s) of other people has been attributed and has been cited and referenced. This thesis is my own work. I have not allowed, and will not allow, anyone to copy my work with the intention of passing it off as his or her own work. Signature ______________________________ Date 06 February 2020 _________________ 1 | Page Abstract The circulation in St Helena Bay and the variability of the retention of the Bay are investigated using seasonal climatologies of the Regional Ocean Modelling System (ROMS). While retention has been studied biologically, the seasonality of the hydrodynamics contributing to the retention have received less attention. -

Derivation of Red Tide Index and Density Using Geostationary Ocean Color Imager (GOCI) Data
remote sensing Article Derivation of Red Tide Index and Density Using Geostationary Ocean Color Imager (GOCI) Data Min-Sun Lee 1,2, Kyung-Ae Park 2,3,* and Fiorenza Micheli 1,4,5 1 Hopkins Marine Station, Stanford University, Pacific Grove, CA 93950, USA; [email protected] (M.-S.L.); [email protected] (F.M.) 2 Department of Earth Science Education, Seoul National University, Seoul 08826, Korea 3 Research Institute of Oceanography, Seoul National University, Seoul 08826, Korea 4 Department of Biology, Stanford University, Stanford, CA 94305, USA 5 Stanford Center for Ocean Solutions, Stanford University, Pacific Grove, CA 93950, USA * Correspondence: [email protected]; Tel.: +82-2-880-7780 Abstract: Red tide causes significant damage to marine resources such as aquaculture and fisheries in coastal regions. Such red tide events occur globally, across latitudes and ocean ecoregions. Satellite observations can be an effective tool for tracking and investigating red tides and have great potential for informing strategies to minimize their impacts on coastal fisheries. However, previous satellite- based red tide detection algorithms have been mostly conducted over short time scales and within relatively small areas, and have shown significant differences from actual field data, highlighting a need for new, more accurate algorithms to be developed. In this study, we present the newly developed normalized red tide index (NRTI). The NRTI uses Geostationary Ocean Color Imager (GOCI) data to detect red tides by observing in situ spectral characteristics of red tides and sea water using spectroradiometer in the coastal region of Korean Peninsula during severe red tide events. The bimodality of peaks in spectral reflectance with respect to wavelengths has become the basis for developing NRTI, by multiplying the heights of both spectral peaks. -

Characteristics of Intermediate Water Flow in the Benguela Current As
Deep-Sea Research II 50 (2003) 87–118 Characteristics of intermediate water flow in the Benguela current as measured with RAFOS floats P.L. Richardsona,*, S.L. Garzolib a Department of Physical Oceanography, Woods Hole Oceanographic Institution, 360 Woods Hole Road, Woods Hole, MA 02543, 3 Water Street, P.O. Box 721, USA b Atlantic Oceanographic and Meteorological Laboratory, NOAA, 4301 Rickenbacker Causeway, Miami, FL 33149, USA Received 28 September 2001; accepted 26 July 2002 Abstract Seven floats (not launched in rings) crossed over the mid-Atlantic Ridge in the Benguela extension with a mean westward velocity of around 2 cm=s between 22S and 35S. Two Agulhas rings crossed over the mid-Atlantic Ridge with a mean velocity of 5:7cm=s toward 2851: This implies they translated at around 3:8cm=s through the background velocity field near 750 m: The boundaries of the Benguela Current extension were clearly defined from the observations. At 750 m the Benguela extension was bounded on the south by 35S and the north by an eastward current located between 18S and 21S. Other recent float measurements suggest that this eastward current originates near the Trindade Ridge close to the western boundary and extends across most of the South Atlantic, limiting the Benguela extension from flowing north of around 20S. The westward transport of the Benguela extension was estimated to be 15 Sv by integrating the mean westward velocities from 22S to 35S and multiplying by the 500 m estimated thickness of intermediate water. Roughly 1.5 Sv of this are transported by the B3 Agulhas rings that cross the mid-Atlantic Ridge each year (as observed with altimetry). -

An Index of Red Tide Mortality on Red Grouper in the Eastern Gulf of Mexico
An Index of Red Tide Mortality on red grouper in the Eastern Gulf of Mexico David Chagaris and Dylan Sinnickson SEDAR61-WP-06 17 August 2018 This information is distributed solely for the purpose of pre-dissemination peer review. It does not represent and should not be construed to represent any agency determination or policy. Please cite this document as: Chagaris, David and Dylan Sinnickson. 2018. An Index of Red Tide Mortality on red grouper in the Eastern Gulf of Mexico. SEDAR61-WP-06. SEDAR, North Charleston, SC. 16 pp. An Index of Red Tide Mortality on red grouper in the Eastern Gulf of Mexico David Chagaris and Dylan Sinnickson University of Florida, IFAS Nature Coast Biological Station and SFRC Fisheries and Aquatic Sciences Program, Gainesville FL. Introduction Karenia brevis is a dinoflagellate that causes toxic red tide blooms in the Gulf of Mexico and Southeast U.S. (Steindinger 2009). In the Gulf of Mexico, red tides occur off the coast of Texas and Florida. They are believed to be caused by a sequence of physical and ecological processes that includes phytoplankton succession stage, nutrient and light availability, and upwelling transport (Walsh et al. 2006). When K. brevis cells lyse they release brevitoxins that are ingested by fish or absorbed across the gill membranes impacting the nervous and respiratory systems and causing mortality (Landsberg 2002). Severe blooms can result in large fish kills (Flaherty and Landsberg 2011; Smith 1975, 1979; Steidinger and Ingle 1972). Decomposition of dead carcasses turns the water hypoxic and releases nutrients that stimulate bloom growth (Walsh et al. -

HARMFUL ALGAL BLOOMS in COASTAL WATERS: Options for Prevention, Control and Mitigation
Science for Solutions A A Special Joint Report with the Decision Analysis Series No. 10 National Fish and Wildlife Foundation onald F. Boesch, Anderson, Rita A dra %: Shumway, . Tesf er, Terry E. February 1997 U.S. DEPARTMENT OF COMMERCE U.S. DEPARTMENT OF THE INTERIOR William M. Daley, Secretary Bruce Babbitt, Secretary The Decision Analysis Series has been established by NOAA's Coastal Ocean Program (COP) to present documents for coastal resource decision makers which contain analytical treatments of major issues or topics. The issues, topics, and principal investigators have been selected through an extensive peer review process. To learn more about the COP or the Decision Analysis Series, please write: NOAA Coastal Ocean Office 1315 East West Highway Silver Spring, MD 209 10 phone: 301-71 3-3338 fax: 30 1-7 13-4044 Cover photo: The upper portion of photo depicts a brown tide event in an inlet along the eastern end of Long Island, New York, during Summer 7986. The blue water is Block lsland Sound. Photo courtesy of L. Cosper. Science for Solutions NOAA COASTAL OCEAN PROGRAM Special Joint Report with the Decision Analysis Series No. 10 National Fish and WildlifeFoundation HARMFUL ALGAL BLOOMS IN COASTAL WATERS: Options for Prevention, Control and Mitigation Donald F. Boesch, Donald M. Anderson, Rita A. Horner Sandra E. Shumway, Patricia A. Tester, Terry E. Whitledge February 1997 National Oceanic and Atmospheric Administration National Fish and Wildlife Foundation D. James Baker, Under Secretary Amos S. Eno, Executive Director Coastal Ocean Office Donald Scavia, Director This ~ublicationshould be cited as: Boesch, Donald F. -

Harmful Algal Blooms (The Florida Red Tide)
FLORIDA RED TIDE What’s New and What’s True Richard H. Pierce, Ph.D. Ecotoxicology and Mote Red Tide Research Team Phytoplankton Ecology; Vince Lovko, Ph.D Environmental Health; Tracy Fanara, Ph.D. Chemical & Physical Ecology; Emily Hall, Ph.D. Coral Reef Monitoring & Assessment; Eric Bartels, MS Ocean Technology; Jim Hillier, Ocean Instrumentation Sp. Mote Research Partners Mote-FWC Cooperative Red Tide Research Partnership; Consistent Base funding for Mote’s Red Tide Research, Mitigation and Control studies Mote-FWC Program Leverage for Additional Research Projects University partners USF, FGCU, FIU, UF, UNCW, Georgia Tech, Ringling, Rutgers, Private Donors & Philanthropic Foundations Topics Mote’s Primary Goals for Red Tide Research: ● Public Health Protection and Information Outreach ● Reducing adverse impacts on public health, coastal marine ecosystems and Florida’s economy. To accomplish these goals, we need to answer the questions: ● What is Red Tide? ● How is Red Tide Harmful? ● What causes Florida Red Tide Events? ● What causes Red Tide to end? ●What can we do about it? FL Red Tide is a Microscopic alga a photosynthetic dinoflagellate (Karenia brevis) causes Harmful Algal Blooms (HABs) 25-35 µm HAB High Concentration of Microscopic Marine Algae That cause Harm two flagella World Wide: Karenia brevis > 2,000 species of marine algae dinoflagellate ~ 200 Species Known Harmful Karenia brevis Phytoplankton culture room Karenia brevis Why is Florida Red Tide Harmful? Produces several Neurotoxins (brevetoxins) Mode of Action: Binds