Spatio-Temporal Variation in Marine Fish Traits Reveals Community- Wide Responses to Environmental Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish) of the Helford Estuary
HELFORD RIVER SURVEY A survey of the Pisces (Fish) of the Helford Estuary A Report to the Helford Voluntary Marine Conservation Area Group funded by the World Wide Fund for Nature U.K. and English Nature P A Gainey 1999 1 Summary The Helford Voluntary Marine Conservation Area (hereafter HVMCA) was designated in 1987 and since that time a series of surveys have been carried out to examine the flora and fauna present. In this study no less that eighty species of fish have been identified within the confines of the HVMCA. Many of the more common fish were found to be present in large numbers. Several species have been designated as nationally scarce whilst others are nationally rare and receive protection at varying levels. The estuary is obviously an important nursery for several species which are of economic importance. A full list of the fish species present and the protection some of them receive is given in the Appendices Nine species of fish have been recorded as new to the HVMCA. ISBN 1 901894 30 4 HVMCA Group Office Awelon, Colborne Avenue Illogan, Redruth Cornwall TR16 4EB 2 CONTENTS Summary Location Map - Fig. 1.......................................................................................................... 1 Intertidal sites - Fig. 2 ......................................................................................................... 2 Sublittoral sites - Fig. 3 ...................................................................................................... 3 Bathymetric chart - Fig. 4 ................................................................................................. -

Florida, Caribbean, Bahamas by Paul Humann and Ned Deloach
Changes in the 4th Edition of Reef Fish Identification - Florida, Caribbean, Bahamas by Paul Humann and Ned DeLoach Prepared by Paul Humann for members of Reef Environmental Education Foundation (REEF) This document summarizes the changes, updates, and new species presented in the 4th edition of Reef Fish Identification- Florida, Caribbean, and Bahamas by Paul Humann and Ned DeLoach (1st printing, released June 2014). This document was created as reference for REEF volunteer surveyors. Chapter 1 – no significant changes Chapter 2 - Silvery pg 64-5t – Redfin Needlefish – new species pg 68-9m – Ladyfish – new species pg 72-3m – Harvestfish – new species pg 72-3b – Longspine Porgy – new species pg74-5 – Silver Porgy & Spottail Pinfish – 2 additional pictures of young and more information on distinguishing between the two species pg 84-5b – Bigeye Mojarra – new species pgs 90-1 & 92-3t – Chubs new species – there are now 4 species of chub in the book – Topsail Chub and Brassy Chub are easily distinguishable. Brassy Chub is the updated ID of Yellow Chub. Bermuda Chub and Gray Chub are lumped due to difficulties in underwater ID (Gray Chub, K biggibus, replaced Yellow Chub in this lumping). Chapter 3 – Grunts & Snappers pg 106-7b and 108-9t – Boga and Bonnetmouth are both now in the Grunt Family (Haemulidae), the Bonnetmouth Family, Inermiidae, is no longer valid. Chapter 4 – Damselfish & Hamlets pgs 132-3 & 134-5 – Cocoa Damselfish and Beaugregory, new pictures and tips for distinguishing between the species pg 142m – Yellowtail Reeffish - new picture of older adult brown variation has been added, which looks of strikingly different from the younger adult pg 147-8b – Florida Barred Hamlet – new species (distinctive from regular Barred Hamlet) pg 153-4t – Tan Hamlet – official species has now been described (Hypoplectrus randallorum), differs from previously included “Tan Hypoplectrus sp.”. -
HELCOM Red List
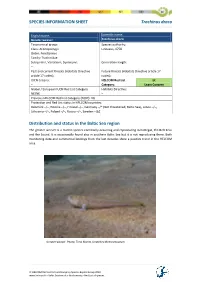
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Age, Growth and Population Dynamics of Lemon Sole Microstomus Kitt(Walbaum 1792)
Age, growth and population dynamics of lemon sole Microstomus kitt (Walbaum 1792) sampled off the west coast of Ireland By Joan F. Hannan Masters Thesis in Fish Biology Galway-Mayo Institute of Technology Supervisors of Research Dr. Pauline King and Dr. David McGrath Submitted to the Higher Education and Training Awards Council July 2002 Age, growth and population dynamics of lemon sole Microstomus kitt (Walbaum 1792) sampled off the west coast of Ireland Joan F. Hannan ABSTRACT The age, growth, maturity and population dynamics o f lemon sole (Microstomus kitt), captured off the west coast o f Ireland (ICES division Vllb), were determined for the period November 2000 to February 2002. The maximum age recorded was 14 years. Males o f the population were dominated by 4 year olds, while females were dominated by 5 year olds. Females dominated the sex ratio in the overall sample, each month sampled, at each age and from 22cm in total length onwards (when N > 20). Possible reasons for the dominance o f females in the sex ratio are discussed. Three models were used to obtain the parameters o f the von Bertalanfly growth equation. These were the Ford-Walford plot (Beverton and Holt 1957), the Gulland and Holt plot (1959) and the Rafail (1973) method. Results o f the fitted von Bertalanffy growth curves showed that female lemon sole o ff the west coast o f Ireland grew faster than males and attained a greater size. Male and female lemon sole mature from 2 years o f age onwards. There is evidence in the population o f a smaller asymptotic length (L«, = 34.47cm), faster growth rate (K = 0.1955) and younger age at first maturity, all o f which are indicative o f a decrease in population size, when present results are compared to data collected in the same area 22 years earlier. -

Collapse and Recovery of Marine Fishes
letters to nature sulphide complexes, indicating that Cu sulphide clusters are more 7. Al-Farawati, R. & van den Berg, C. M. G. Metal-sulfide complexation in seawater. Mar. Chem. 63, 331–352 (1999). stable than Cu organic complexes. This explains why laboratory 8. Luther III, G. W., Rickard, D. T., Theberge, S. M. & Olroyd, A. Determination of metal (bi)sulfide cultures of oceanic phytoplankton have been observed to increase stability constants of Mn2+,Fe2+,Co2+,Ni2+,Cu2+, and Zn2+ by voltammetric methods. Environ. Sci. the production of total dissolved sulphides when the concentrations Technol. 30, 671–679 (1996). of free Cu and Zn in the culture media were increased22. Although 9. Helz, G. R, Charnock, J. M., Vaughan, D. J. & Garner, C. D. Multinuclearity of aqueous copper and zinc bisulfide complexes—an EXAFS investigation. Geochim. Cosmochim. Acta 57, the data that we report here suggest that metal sulphide formation is 15–25 (1993). a means of detoxifying trace metals for organisms, further toxico- 10. Luther III, G. W., Theberge, S. M. & Rickard, D. T. Evidence for aqueous clusters as intermediates logical studies are needed to quantify the roles both sulphides and during zinc sulfide formation. Geochim. Cosmochim. Acta 19/20, 3159–3169 (1999). ‘natural’ organic ligands play in controlling Cu toxicity in natural 11. Peters, J. W., Lanzilotta, W. N., Lemon, B. J. & Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853– waters. 1858 (1998). Sulphur complexation may have a dramatic effect on the acute 12. -

Sol E I Da E of the Eastern North Atlantic
FICHES D’IDENTIFICATION DU ZOOPLANCTON EditCes par J. H. FRASER Marine Laboratory, P.O. Box 101, Victoria Road, Aberdeen AB9 8DB, Scotland FICHE NO. 1501151 SOL E I DA E OF THE EASTERN NORTH ATLANTIC J. H. Nichols Fisheries Laboratory Lowestoft, Suffolk NR 33 OHT, England (This sheet may be referred to in the following form: Nichols, J. H. 1976. Soleidae, Fich. Ident. Zooplancton 150/151: .10 pp.) https://doi.org/10.17895/ices.pub.5120 Conseil International pour 1’Exploration de la Mer Charlottenlund Slot, DK-2920 Charlottenlund Danemark SEPTEMBRE 1976 2 INTRODUCTION This sheet deals in detail with the four species of Soleidae occurring commonly within the areas listed in Table 1. The Soleidae are a warm- water family, reaching the northerly limit of their distribution in the northern North Sea. Consequently, many more genera are found south of the Atlantic area covered by Table 1, and in the Mediterranean. The adult of one of the southerly genera Dicologlossu uzeviu, has been recorded once in the western English Channel and it also occurs occasionally in the southern part of the Bay of Biscay. Insufficient information on the egg and larval development of this genus, and the rare deep-water form Buthysoleu profondicolu, is available for them to be included here. This sheet should be used together with no. 4-6, (NICHOLS,1971), on the Pleuronectidae, which includes a key to the families in the order Hetero- somata. The information on material and methods in that sheet is also applicable here. Since its publication I have found the use of a polarized light system on a steroscopic microsccpe extremely useful for counting vertebrae and myotomes when these are necessary. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Appendix 13.2 Marine Ecology and Biodiversity Baseline Conditions
THE LONDON RESORT PRELIMINARY ENVIRONMENTAL INFORMATION REPORT Appendix 13.2 Marine Ecology and Biodiversity Baseline Conditions WATER QUALITY 13.2.1. The principal water quality data sources that have been used to inform this study are: • Environment Agency (EA) WFD classification status and reporting (e.g. EA 2015); and • EA long-term water quality monitoring data for the tidal Thames. Environment Agency WFD Classification Status 13.2.2. The tidal River Thames is divided into three transitional water bodies as part of the Thames River Basin Management Plan (EA 2015) (Thames Upper [ID GB530603911403], Thames Middle [ID GB53060391140] and Thames Lower [ID GB530603911401]. Each of these waterbodies are classified as heavily modified waterbodies (HMWBs). The most recent EA assessment carried out in 2016, confirms that all three of these water bodies are classified as being at Moderate ecological potential (EA 2018). 13.2.3. The Thames Estuary at the London Resort Project Site is located within the Thames Middle Transitional water body, which is a heavily modified water body on account of the following designated uses (Cycle 2 2015-2021): • Coastal protection; • Flood protection; and • Navigation. 13.2.4. The downstream extent of the Thames Middle transitional water body is located approximately 12 km downstream of the Kent Project Site and 8 km downstream of the Essex Project Site near Lower Hope Point. Downstream of this location is the Thames Lower water body which extends to the outer Thames Estuary. 13.2.5. A summary of the current Thames Middle water body WFD status is presented in Table A13.2.1, together with those supporting elements that do not currently meet at least Good status and their associated objectives. -

Aqua, International Journal of Ichthyology AQUA19(1):AQUA 24/01/13 12:37 Pagina 1
AQUA19(1):AQUA 24/01/13 12:37 Pagina 101 aqua International Journal of Ichthyology Vol. 19 (1), 21 January 2013 Aquapress ISSN 0945-9871 AQUA19(1):AQUA 24/01/13 12:37 Pagina 102 aqua - International Journal of Ichthyology Managing Editor: Scope aqua is an international journal which publishes original Heiko Bleher scientific articles in the fields of systematics, taxonomy, Via G. Falcone 11, bio geography, ethology, ecology, and general biology of 27010 Miradolo Terme (PV), Italy fishes. Papers on freshwater, brackish, and marine fishes Tel. & Fax: +39-0382-754129 will be considered. aqua is fully refereed and aims at pub- E-mail: [email protected] lishing manuscripts within 2-4 months of acceptance. In www.aqua-aquapress.com view of the importance of color patterns in species identi - fication and animal ethology, authors are encouraged to submit color illustrations in addition to descriptions of Scientific Editor: coloration. It is our aim to provide the international sci- entific community with an efficiently published journal Frank Pezold meeting high scientific and technical standards. College of Science & Engineering Texas A&M University – Corpus Christi Call for papers 6300 Ocean Drive – Corpus Christi, TX 78412-5806 The editors welcome the submission of original manu- Tel. 361-825-2349 scripts which should be sent in digital format to the scien- E-mail: [email protected] tific editor. Full length research papers and short notes will be considered for publication. There are no page charges and color illustrations will be published free of charge. Authors will receive one free copy of the issue in which Editorial Board: their paper is published and an e-print in PDF format. -

Inventory of Tidepool and Estuarine Fishes in Acadia National Park
INVENTORY OF TIDEPOOL AND ESTUARINE FISHES IN ACADIA NATIONAL PARK Edited by Linda J. Kling and Adrian Jordaan School of Marines Sciences University of Maine Orono, Maine 04469 Report to the National Park Service Acadia National Park February 2008 EXECUTIVE SUMMARY Acadia National Park (ANP) is part of the Northeast Temperate Network (NETN) of the National Park Service’s Inventory and Monitoring Program. Inventory and monitoring activities supported by the NETN are becoming increasingly important for setting and meeting long-term management goals. Detailed inventories of fishes of estuaries and intertidal areas of ANP are very limited, necessitating the collection of information within these habitats. The objectives of this project were to inventory fish species found in (1) tidepools and (2) estuaries at locations adjacent to park lands on Mount Desert Island and the Schoodic Peninsula over different seasons. The inventories were not intended to be part of a long-term monitoring effort. Rather, the objective was to sample as many diverse habitats as possible in the intertidal and estuarine zones to maximize the resultant species list. Beyond these original objectives, we evaluated the data for spatial and temporal patterns and trends as well as relationships with other biological and physical characteristics of the tidepools and estuaries. For the tidepool survey, eighteen intertidal sections with multiple pools were inventoried. The majority of the tidepool sampling took place in 2001 but a few tidepools were revisited during the spring/summer period of 2002 and 2003. Each tidepool was visited once during late spring (Period 1: June 6 – June 26), twice during the summer (Period 2: July 3 – August 2 and Period 3: August 3 – September 18) and once during early fall (Period 4: September 29 – October 21). -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores.