Write the Balanced Equation for Beryllium Iodide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compound Formula Tin (II) Nitride Silver Oxide Lithium Sulfide Magnesium Sulfide
Ionic Bonding Drill Write the correct formula for the following compounds Compound Formula tin (II) nitride silver oxide lithium sulfide magnesium sulfide copper (I) nitride AgCl boron iodide potassium fluoride copper (I) chloride is CuCl iron (II) oxide is FeO tin (IV) fluoride is SnF4 nickel (II) fluoride is NiF2 lead (IV) oxide is PbO2 silver chloride is calcium iodide is CaI2 potassium bromide sodium phosphide iron (II) chloride copper (I) bromide lead (II) sulfide lead (IV) nitride beryllium nitride potassium bromide is KBr sodium phosphide is Na3P iron (II) chloride is FeCl2 copper (I) bromide is CuBr lead (II) sulfide is PbS lead (IV) nitride is Pb3N4 beryllium nitride is Be3N2 copper (I) chloride iron (II) oxide tin (IV) fluoride nickel (II) fluoride lead (IV) oxide Ag2O silver chloride calcium iodide Answers copper (I) nitride is Cu3N boron iodide is BI3 potassium fluoride is KF silver oxide is lithium sulfide is Li2S magnesium sulfide is MgS tin (II) nitride is Sn3N2 Ionic Bonding Drill Write the correct formula for the following compounds Compound Formula lithium bromide sodium sulfide lead (II) chloride nickel (II) oxide AlBr3 copper (II) oxide AlI3 iron (II) fluoride tin (II) oxide iron (II) oxide is FeO lead (II) oxide is PbO aluminum bromide is potassium oxide is K2O potassium oxide is K2O aluminum iodide is lead (II) nitride is Pb3N2 tin (IV) sulfide iron (III) sulfide lead (II) nitride copper (II) oxide silver fluoride AgF sodium chloride magnesium bromide tin (IV) sulfide is SnS2 iron (III) sulfide is Fe2S3 lead (II) -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Mole Calculation Worksheet – Answer Key
Name_______________________ Date___________________ Per___________ Mole to Grams, Grams to Moles Conversions Worksheet What are the molecular weights of the following compounds? (all masses must be to nearest hundredth) 1) NaOH 2) H3PO4 3) H2O 4) Mn2Se7 5) MgCl2 6) (NH4)2SO4 There are three definitions (equalities) of mole. They are: 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 L of a gas at STP (You do not need to worry about this yet) Each definition can be written as a set of two conversion factors. They are: 1 mole = molar mass(g) can be written as ____1 mole OR _molar mass (g) molar mass (g) 1 mole 1 mole = 6.02 x 1023 particles can be written as ____1 mole OR __6.02 x 1023_ 6.02 x 1023 1 mole Solve the following: 1) How many moles are in 15 grams of lithium? (molar mass of lithium is 6.94 g/mole) 15 grams x 1 mole = 2.1614 moles lithium = 2.2 mol es Li 6.94grams 2) How many grams are in 2.4 moles of sulfur? (molar mass of sulfur is 32.07 g/ mole) 2.4 moles x 32.07 grams = 76.97 grams sulfur = 77 g Sulfur 1 mole 3) How many moles are in 22 grams of argon? 4) How many grams are in 88.1 moles of magnesium? 5) How many moles are in 2.3 grams of phosphorus? 6) How many grams are in 11.9 moles of chromium? 7) How many moles are in 9.8 grams of calcium? 8) How many grams are in 238 moles of arsenic? Solve the following: 9) How many grams are in 4.5 moles of sodium fluoride, NaF? (molar mass of NaF is 22.99 + 19.00 = 41.99 g/ mole) 4.5 moles x 41.99 grams = -

"Sony Mobile Critical Substance List" At
Sony Mobile Communications Public 1(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Prepared by Date SEM/CGQS JOHAN K HOLMQVIST 2014-02-20 Contents responsible if other than preparer Remarks This document is managed in metaDoc. Approved by SEM/CGQS (PER HÖKFELT) Sony Mobile Critical Substances Second Edition Contents 1 Revision history.............................................................................................3 2 Purpose.........................................................................................................3 3 Directive ........................................................................................................4 4 Application ....................................................................................................4 4.1 Terms and Definitions ...................................................................................4 5 Comply to Sony Technical Standard SS-00259............................................6 6 The Sony Mobile list of Critical substances in products................................6 7 The Sony Mobile list of Restricted substances in products...........................7 8 The Sony Mobile list of Monitored substances .............................................8 It is the responsibility of the user to ensure that they have a correct and valid version. Any outdated hard copy is invalid and must be removed from possible use. Public 2(16) DIRECTIVE Document number Revision 6/034 01-LXE 110 1408 Uen C Substance control Sustainability is the backbone -
Printed Copies for Reference Only
Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 APPROVERS INFORMATION PREPARED BY: Jennilyn Rivera Dinglasan Title: EHS DATE: 5/3/2017 2:35:30 AM APPROVED BY: Jennilyn DATE: 8/10/2017 7:00:05 Rivera Dinglasan Title: EHS PM DATE: 7/21/2017 2:43:17 APPROVED BY: Aline Zeng Title: Purchasing AM APPROVED BY: Bill Hemrich Title: Purchasing DATE: 6/1/2017 3:28:35 PM DATE: 6/11/2017 11:05:48 APPROVED BY: Jade Yuan Title: Purchasing PM APPROVED BY: Matthew DATE: 5/26/2017 6:54:14 Briggs Title: Purchasing AM DATE: 5/26/2017 3:56:07 APPROVED BY: Michael Ji Title: Purchasing AM DATE: 8/31/2017 4:24:35 APPROVED BY: Olga Chen Title: Purchasing AM APPROVED BY: Alfredo DATE: 6/2/2017 11:54:20 Heredia Title: Supplier Quality AM APPROVED BY: Audrius DATE: 5/31/2017 1:20:01 Sutkus Title: Supplier Quality AM DATE: 5/26/2017 2:03:53 APPROVED BY: Sam Peng Title: Supplier Quality AM APPROVED BY: Arsenio DATE: 6/28/2017 2:43:49 Mabao Cesista Jr. Title: EHS Manager AM Printed copies for reference only Printed copies for reference only Supplier Environmental Health and Safety Number:CHI-EHS30-000 Revision:A Specification 1 1.0 Purpose and Scope 1.1 This specification provides general requirements to suppliers regarding Littelfuse Inc’s EHS specification with regards to regulatory compliance, EHS management systems, banned and restricted substances, packaging, and product environmental content reporting. 1.2 This specification applies to all equipment, materials, parts, components, packaging, or products supplied to Littelfuse, Inc. -

CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula
CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice: Complete these in lab and on your own time for practice. You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following: 1. sulfur dioxide ____SO2______________ 26. methane ____CH4_____________ 2. sodium thiosulfate ____Na2S2O3__________ 27. copper (II) sulfate ____CuSO4___________ 3. ammonium phosphate ____(NH4)3PO4_________ 28. nitrogen dioxide ____NO2_____________ 4. potassium chlorate ____KClO3____________ 29. mercury (II) chloride ____HgCl2____________ 5. lithium hydroxide ____LiOH_____________ 30. tin (II) bromide ____SnBr2____________ 6. zinc nitrite ____Zn(NO2)2__________ 31. silver iodide ____AgI______________ 7. sodium sulfate ____Na2SO4____________ 32. magnesium bisulfite ____Mg(HSO3)2________ 8. cobalt (IV) bisulfite ____Co(HSO3)4_________ 33. carbon disulfide ____CS2______________ 9. cadmium nitrate ____Cd(NO3)2__________ 34. beryllium periodate ____Be(IO4)2__________ 10. nitric oxide ____NO_______________ 35. platinum (IV) cyanide ____Pt(CN)4___________ 11. hydrogen peroxide ____H2O2______________ 36. ammonia ____NH3______________ 12. carbon monoxide ____CO_______________ 37. dinitrogen oxide ____N2O______________ 13. silicon dioxide ____SiO2______________ 38. ferric oxide ____Fe2O3____________ 14. copper (I) bromide ____CuBr______________ 39. gold (III) chloride ____AuCl3____________ 15. iron (II) chromate ____FeCrO4____________ 40. strontium sulfide ____SrS______________ 16. mercury (I) fluoride -

Li Na K Be Mg Ca B Al Ni Au Ag
LAST NAME____________________ FIRST NAME____________________________ DATE ___________ 6.1 NAMING IONIC COMPOUNDS = Use the notes to write the correct names of the IONIC COMPOUNDS created by the Cations and Anions. SIMPLE EXAMPLES Anions Cations ↓ F Cl Br I Li Lithium Fluoride Lithium Chloride Lithium Bromide Lithium Iodide Na Sodium Fluoride Sodium Chloride Sodium Bromide Sodium Iodide K Potassium Fluoride Potassium Chloride Potassium Bromide Potassium Iodide Be Beryllium Fluoride Beryllium Chloride Beryllium Bromide Beryllium Iodide Mg Magnesium Fluoride Magnesium Chloride Magnesium Bromide Magnesium Iodide Ca Calcium Fluoride Calcium Chloride Calcium Bromide Calcium Iodide B Boron Fluoride Boron Chloride Boron Bromide Boron Iodide Al Aluminum Fluoride Aluminum Chloride Aluminum Bromide Aluminum Iodide Ni Nickel Fluoride Nickel Chloride Nickel Bromide Nickel Iodide Au Gold Fluoride Gold Chloride Gold Bromide Gold Iodide Ag Silver Fluoride Silver Chloride Silver Bromide Silver Iodide And some more Anions Cations ↓ O S N Li Lithium Oxide Lithium Sulfide Lithium Nitride Na Sodium Oxide Sodium Sulfide Sodium Nitride K Potassium Oxide Potassium Sulfide Potassium Nitride Be Beryllium Oxide Beryllium Sulfide Beryllium Nitride Mg Magnesium Oxide Magnesium Sulfide Magnesium Nitride Ca Calcium Oxide Calcium Sulfide Calcium Nitride B Boron Oxide Boron Sulfide Boron Nitride Al Aluminum Oxide Aluminum Sulfide Aluminum Nitride Ni Nickel Oxide Nickel Sulfide Nickel Nitride Au Gold Oxide Gold Sulfide Gold Nitride Ag Silver Oxide Silver Sulfide Silver -

Hazardous Waste Management Guidebook
Hazardous Waste Management Guidebook FOR UNIVERSTIY AT BUFFALO CAMPUS LABORATORIES Prepared By Environment, Health & Safety Services 220 Winspear Avenue Buffalo, NY 14215 Phone: 716-829-3301 Web: www.ehs.buffalo.edu UB EH&S Hazardous Waste Management Guidebook Table of Contents 1.0 PURPOSE ........................................................................................... 3 2.0 SCOPE ............................................................................................... 4 3.0 DEFINITIONS ...................................................................................... 4 4.0 RESPONSIBILITIES ............................................................................... 5 4.1 EH&S ................................................................................................... 5 4.2 Faculty, Staff, and Students ............................................................. 5 5.0 PROCEDURES ....................................................................................... 6 5.1 Hazardous Waste Determination ................................................... 6 5.1.1 Characteristic Hazardous Wastes .......................................... 7 5.1.2 Listed Hazardous Wastes ........................................................ 9 5.2 Satellite Accumulation of Hazardous Waste ............................ 9 5.2.1 Accumulation Areas ............................................................. 10 5.2.2 Requirements for Hazardous Waste Containers ................ 10 5.2.3 Segregation of Hazardous Wastes ..................................... -
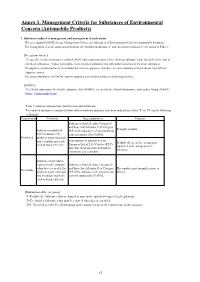
Annex 1. Management Criteria for Substances of Environmental Concern (Automobile Products)
Annex 1. Management Criteria for Substances of Environmental Concern (Automobile Products) 1. Substances subject to management and management classifications We have adopted GADSL for our Management Criteria for Substances of Environmental Concern (Automobile Products). The management classifications and definitions for "prohibited substances" and "declarable substances" are shown in Table 1. [Precautions for use] The specific chemical substances within GADSL only enumerate some of the chemical substances that fall under their class of chemical substances. It does not regulate every chemical substance that falls under said class of chemical substances. The Japanese translation has been included for reference purposes, but there are some substances which do not have official Japanese names. The latest information on GADSL must be obtained and confirmed from the following website. [GADSL] The Global Automotive Declarable Substance List (GADSL) released by the Global Automotive Stakeholder Group (GASG) http://www.gadsl.org/ Table 1. Stanley's management classifications and definitions The reported substances contained within vehicle materials and parts have been indicated as either "P" or "D" via the following definitions. Classification Definition Target substances Response Substances that fall under Category P and those that fall under P of Category Promptly prohibit. Substances prohibited D/P of the substances of environmental from inclusion in the concern stipulated by GADSL Prohibited products, parts, materials, Four substances stipulated in the and secondary materials Prohibit all except for exempt parts European End of Life Vehicles (ELV) used at Stanley Electric stipulated in the European ELV Directive (lead, mercury, hexavalent Directive. chromium, and cadmium) Substances that must be reported to the company Substances that fall under Category D when they are used in the and those that fall under D of Category The supplier must promptly report to Declarable products, parts, materials, D/P of the substances of environmental Stanley. -

US5359094.Pdf
|||||||||||||I|| US005359094A United States Patent 19 11 Patent Number: 5,359,094 Teles et al. 45) Date of Patent: Oct. 25, 1994 (54) PREPARATION OF GLYCERYL 3,379,693 4/1968 Hostettler et al. .................. 549/229 CARBONATE 4,231,937 1/1980 Kao et al. ............ ... 549/229 4,314,945 2/1982 McMullen et al. ... 549/229 75 Inventors: Joaquim H. Teles, Ludwigshafen; 4,344,881 8/1982 Strege et al. ..... ... 549/229 Norbert Rieber, Mannheim; 4,483,994 11/1984 Jacobson ...... ... 549/229 Wolfgang Harder, Weinheim, all of 4,658,041 4/1987 Renga .......... ... 549/229 Fed. Rep. of Germany 4,835,289 5/1989 Brindapke ... ... 549/229 73) Assignee: BASF Aktiengesellschaft, 5,091,543 2/1992 Grey .................... ... 549/229 Ludwigshafen, Fed. Rep. of 5, 18,818, 6/1992 Delledonine et al. ............... 549/229 Germany FOREIGN PATENT DOCUMENTS (21) Appl. No.: 99,142 2222488 11/1972 Fed. Rep. of Germany. 2265228 12/1976 Fed. Rep. of Germany . 22 Filed: Jul. 29, 1993 1382313 1/1975 United Kingdom . 30 Foreign Application Priority Data Primary Examiner-Cecilia Tsang Aug. 5, 1992 (DE Fed. Rep. of Germany ....... 4225870 Attorney, Agent, or Firm-Keil & Weinkauf 51 Int. Cl. ............................................ CO7D 317/36 (57) ABSTRACT 52 U.S. C. .................................... 549/228; 549/229; 549/230 A process for the preparation of glyceryl carbonate by 58) Field of Search ........................ 549/228, 229, 230 causing glycerol to react with carbon monoxide and oxygen in the presence of a Group Ib, Group IIb, or (56) References Cited Group VIIIb catalyst as per the Periodic Table attem U.S. -

3TR-ANGELL.Txt 1 INTERVIEW of RONALD PAUL ANGELL 2 3 4 Q
3TR-ANGELL.txt 1 INTERVIEW OF RONALD PAUL ANGELL 2 3 4 Q. This is Deborah Tavares with StoptheCrime.net. 5 Today is May 15, 2014. I'm happy to introduce my guest 6 this evening. This is Ronald Paul Angell. And we're 7 going to be discussing the topic of global catastrophe 8 and the need for a global wake-up call, and about why 9 chemtrails exist, why the populations globally are 10 experiencing increased diseases, and what you need to 11 know about these calamities that we all face. 12 So I want to welcome you, Ron, to the program. Page 1 3TR-ANGELL.txt 13 A. Thank you. 14 Q. And if you would just like to cover this 15 global catastrophe, and talk about why chemtrails exist, 16 and why we're experiencing heightened levels of diseases 17 and immune assaults upon our systems. 18 A. Okay. Well, it gets back to a paper that is 19 talking about directed-energy radiation. That is the 20 missing link, you could say, as to why the chemtrails 21 are being used along with the EMF radiation from the 22 cell towers, the GWEN towers, the radio transmission 23 towers, the weaponized power grid-Smart meters, cell 24 phones, street lights, energy-saving light bulbs, street 25 signal crossings, Energy Star appliances and the 1 Page 2 3TR-ANGELL.txt 1 satellites. 2 And this U.C. Berkeley paper from the Research 3 Medicine and Radiation Biophysics Division, Lawrence 4 Berkeley Laboratory, University of California, Berkeley, 5 California, 94720, was put out July 1991.