Prunus Serotina
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pru Nus Contains Many Species and Cultivars, Pru Nus Including Both Fruits and Woody Ornamentals
;J. N l\J d.000 A~ :J-6 '. AGRICULTURAL EXTENSION SERVICE UNIVERSITY OF MINNESOTA • The genus Pru nus contains many species and cultivars, Pru nus including both fruits and woody ornamentals. The arboretum's Prunus maacki (Amur Cherry). This small tree has bright, emphasis is on the ornamental plants. brownish-yellow bark that flakes off in papery strips. It is par Prunus americana (American Plum). This small tree furnishes ticularly attractive in winter when the stems contrast with the fruits prized for making preserves and is also an ornamental. snow. The flowers and fruits are produced in drooping racemes In early May, the trees are covered with a "snowball" bloom similar to those of our native chokecherry. This plant is ex of white flowers. If these blooms escape the spring frosts, tremely hardy and well worth growing. there will be a crop of colorful fruits in the fall. The trees Prunus maritima (Beach Plum). This species is native to the sucker freely, and unless controlled, a thicket results. The A coastal plains from Maine to Virginia. It's a sprawling shrub merican Plum is excellent for conservation purposes, and the reaching a height of about 6 feet. It blooms early with small thickets are favorite refuges for birds and wildlife. white flowers. Our plants have shown varying degrees of die Prunus amygdalus (Almond). Several cultivars of almonds back and have been removed for this reason. including 'Halls' and 'Princess'-have been tested. Although Prunus 'Minnesota Purple.' This cultivar was named by the the plants survived and even flowered, each winter's dieback University of Minnesota in 1920. -

Biscogniauxia Granmoi (Xylariaceae) in Europe
©Österreichische Mykologische Gesellschaft, Austria, download unter www.biologiezentrum.at Osten. Z.Pilzk 8(1999) 139 Biscogniauxia granmoi (Xylariaceae) in Europe THOMAS L£SS0E CHRISTIAN SCHEUER Botanical Institute, Copenhagen University Institut fiir Botanik der Karl-Franzens-Universitat Oster Farimagsgade 2D Holteigasse 6 DK-1353 Copenhagen K, Denmark A-8010 Graz, Austria e-mail: [email protected] e-mail: [email protected] ALFRED GRANMO Trornso Museum, University of Tromse N-9037 Tromso, Norway e-mail: [email protected] Received 5. 7. 1999 Key words: Xylariaceae, Biscogniauxia. - Taxonomy, distribution. - Fungi of Europe, Asia. Abstract: Biscogniauxia granmoi, growing on Prunus padus (incl. var. pubescens = Padus asiatica) is reported from Europe and Asia, with material from Austria, Latvia, Norway, Poland, and Far Eastern Russia. It is compared with B. nummulana s. str., B. capnodes and B. simphcior. The taxon was included in the recent revision of Biscogniauxia by JU & al. {1998, Mycotaxon 66: 50) under the name "B. pruni GRANMO, L/ESS0E & SCHEUER" nom. prov. Zusammenfassung: Biscogniauxia granmoi, die bisher ausschließlich auf Prunus padus (inkl. var pubescens = Padus asiatica) gefunden wurde, wird aufgrund von Aufsammlungen aus Europa und Asien vorgestellt. Die bisherigen Belege stammen aus Österreich, Litauen, Norwegen, Polen und dem femöstlichen Teil Rußlands. Die Unterschiede zu B. nummulana s. Str., B. capnodes und H simphaor werden diskutiert. Dieses Taxon wurde unter dem Namen "B. pruni Granmo, l.aessoe & Scheuer" nom. prov. schon von JU & al. (1998, Mycotaxon 66: 50) in ihre Revision der Gattung Biscogniauxia aufge- nommen. The genus Biscogniauxia KUNTZE (Xylariaceae) was resurrected and amended by POUZAR (1979, 1986) for a group of Xylariaceae with applanate dark stromata that MILLER (1961) treated in Hypoxylon BULL., and for a group of species with thick, discoid stromata formerly placed in Nummularia TUL. -

Black Cherry (Prunus Serotina Ehrh.)
DOCS A 13.31:C 424/971 .: z,' BLACK CHERRY Black cherry, also commonly called cherry, grows in significant commercial quantities only in the north- ern Allegheny Mountains. Cherry wood is reddish and takes a lustrous finish; it is a prized furniture wood and brings high prices in veneer log form. lt is straight-grained moderately hard, and stable; it can be machined easily. Black cherry is widely used in the printing industries to mount engravings, elec- trotypes, and zinc etchings. lt is also used for wall paneling, flooring, patterns, professional and scien- tific instruments, handles, and other specialty items. -"3242, /9 \(j\\ '-' 'Li \c1 - - / U.S. Department of Agriculture Forest Service . American Woods-FS 229 Revised February 1971 BLACK CHERRY (Prunus sero tina Ehrh.) Charles J. Gatchell1 DISTRiBUTION Florida west to eastern Texas, north to central Minne. z Black cherry and its varieties grow under a wide sota, and east through northern Michigan, Ontario, range of climatic conditions (fig. 1). It is found prin. and Quebec to Maine and Nova Scotia. It is also found cipally throughout the eastern half of the United States, in scattered locations in Arizona, New Mexico, western from the Plains to the Atlantic, and the Great Lakes to Texas, Guatemala, and Mexico. It grows extensively in the Gulf of Mexico. Its range extends from northern western and central Mexico. Research forest products technologist, Northeastern Forest Black cherry is of commercial significance only in Experiment Station, USDA Forest Service, a narrow area centering in western Pennsylvania. Major 0 ?_ .90.?0 3?0. 95 F-506642 Figure 1.-Natural range of black cherry, Prunus serotina. -

Production, Pomological and Nutraceutical Properties of Apricot
1 Production, pomological and nutraceutical properties of apricot Khaled Moustafa1* and Joanna Cross2 1Editor of ArabiXiv (arabixiv.org), Paris, France 2Nigde Omer Halisdemir University, Nigde, Turkey Correspondence: [email protected] Abstract Apricot (Prunus sp.) is an important fruit crop worldwide. Despite recent advances in apricot research, much is still to be done to improve its productivity and environmental adaptability. The availability of wild apricot germplasms with economically interesting traits is a strong incentive to increase research panels toward improving its economic, environmental and nutritional characteristics. New technologies and genomic studies have generated a large amount of raw data that the mining and exploitation can help decrypt the biology of apricot and enhance its agronomic values. Here, we outline recent findings in relation to apricot production, pomological and nutraceutical properties. In particular, we retrace its origin from central Asia and the path it took to attain Europe and other production areas around the Mediterranean basin while locating it in the rosaceae family and referring to its genetic diversities and new attempts of classification. The production, nutritional, and nutraceutical importance of apricot are recapped in an easy readable and comparable way. We also highlight and discuss the effects of late frost damages on apricot production over different growth stages, from swollen buds to green fruits formation. Issues related to the length of production season and biotic and abiotic environmental challenges are also discussed with future perspective on how to lengthen the production season without compromising the fruit quality and productivity. Keywords Apricot kernel oil, plum pox virus, prunus armeniaca, spring frost, stone fruit, sharka. -

Ships of 8 Tree Species in Navajo National Monument, Arizona
Population Dynamics and Age Relation- ships of 8 Tree Species in Navajo National Monument, Arizona J.D. BROTHERSON, S.R. RUSHFORTH, W.E. EVENSON, J.R. JOHANSEN, AND C. MORDEN The presence of 3 major archeological ruins dating from the 1 lth cent slickrock areas and the plateau behind the canyon rim to 13th centuries (Woodbury 1963) provided the primary motiva- (Brotherson et al. 1978). The pigmy woodland community covers tion for including Navajo National Monument in the National more area than any other type in northeastern Arizona. Parks System. Also included in the monument are some unique Gambel oak is the most extensive type in the Monument aside ecosystems, especially a small relict of “mountain vegetation” from tne pinyon-juniper community. It is found inall 3 segments of found in Betatakin Canyon. As visitor pressures mount annually, the monument but reaches its greatest development at Keet Steel proper management of these unique ecosystems becomes highly and Betatakin. Pnrnus emarginata (bittercherry) also grows at important. Since trees are the dominant features of these ecosys- Keet Steel but is uncommon. tems, and are central to management considerations, the present The streamside community of the Inscription House segment of work has examined populations of 8 major tree species in the the monument contains Popufus fremontii (Fremont poplar), monument. Populus angustifolia (narrowleaf cottonwood), Salk goodingii The objectives of this study were, first, to develop age prediction (Gooding willow), Tamarix ramosissima (salt cedar), and Elaeg- equations for tree species growing in Navajo National Monument, nus angustifolia (Russian olive). Fremont poplar is the dominant and second, to assess the present age profiles, reproductive recruit- tree within this community with salt cedar attaining local impor- ment, and density relationships of these tree populations. -

Comparing Negative Impacts of Prunus Serotina, Quercus Rubra and Robinia Pseudoacacia on Native Forest Ecosystems
Article Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems Rodolfo Gentili 1,* , Chiara Ferrè 1, Elisa Cardarelli 2, Chiara Montagnani 1, Giuseppe Bogliani 2 , Sandra Citterio 1 and Roberto Comolli 1 1 Department of Earth and Environmental Sciences, University of Milano-Bicocca, Piazza della Scienza 1, 20126 Milano, Italy; [email protected] (C.F.); [email protected] (C.M.); [email protected] (S.C.); [email protected] (R.C.) 2 Department of Earth and Environmental Science, University of Pavia, Via Ferrata 9, 27100 Pavia, Italy; [email protected] (E.C.); [email protected] (G.B.) * Correspondence: [email protected]; Tel.: +39-02-6448-2700 Received: 12 August 2019; Accepted: 23 September 2019; Published: 26 September 2019 Abstract: The introduction of invasive alien plant species (IAPS) can modify plant-soil feedback, resulting in an alteration of the abiotic and biotic characteristics of ecosystems. Prunus serotina, Quercus rubra and Robinia pseudoacacia are IAPS of European temperate forests, where they can become dominant and suppress the native biodiversity. Assuming that the establishment of these invasive species may alter native forest ecosystems, this study comparatively assessed their impact on ecosystems. This study further investigated plant communities in 12 forest stands, dominated by the three IAPS and native trees, Quercus robur and Carpinus betulus (three plots per forest type), in Northern Italy, and collected soil samples. The relationships between the invasion of the three IAPS and modifications of humus forms, soil chemical properties, soil biological quality, bacterial activity and plant community structure and diversity (α-, β-, and γ-diversity) were assessed using one-way ANOVA and redundancy analyses (RDA). -
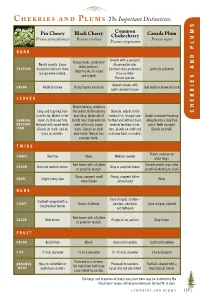
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

TREES for WESTERN NEBRASKA Justin Evertson & Bob Henrickson
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS TREES FOR WESTERN NEBRASKA Justin Evertson & Bob Henrickson. For more plant information, visit plantnebraska.org or retreenbraska.unl.edu The following species are recommended for areas in the western half of Nebraska and/or typically receive less than 20” of moisture per year. Size Range: The size range indicated for each plant is the expected average mature height x spread for Nebraska. Large Deciduous Trees (typically over 40 feet tall at maturity) NOTE ON ASH SPECIES: Native American ash trees are being decimated by Emerald Ash Borer (EAB) and the insect is now in Nebraska. NSA recommends that native ash species no longer be planted in Nebraska. 1. Ash, Manchurian - Fraxinus mandshurica (from Asia; upright growth; drought tolerant; may be resistant to EAB; 40’x 30’) 2. Catalpa, Northern - Catalpa speciosa (native; tough tree; large, heart-shaped leaves, showy flowers and long seed pods; 50’x 35’) 3. Coffeetree, Kentucky - Gymnocladus dioicus (native; amazingly adaptable; beautiful winter form; 50’x 40’) 4. Cottonwood, Eastern - Populus deltoides (majestic native; not for extremely dry sites; avoid most cultivars; 80’x 60’) 5. Cottonwood, Lanceleaf - Populus acuminata (native; naturally occurring hybrid; narrow leaves; for west. G.P.; 50’x 35’) 6. Elm, American - Ulums americana (disease resistant varieties include ‘Valley Forge’ and ‘New Harmony’; 50’x50’) 7. Elm, Japanese - Ulmus davidiana var. japonica (cold tolerant; rounded; glossy green; ‘Discovery’ is a cultivar from Manitoba Canada; 45’x 45’) 8. Elm, Rock - Ulmus thomasii (distinctive corky stems; upright habit; DED resistance in west; 50-60’x 30-40’) New Elm, Hybrids - many disease resistant hybrid elms have been developed and show promise, including: 9. -

Black Cherry Rosaceae Rose Family David A
Prunus serotina Ehrh. Black Cherry Rosaceae Rose family David A. Marquis Black cherry (Prunus serotinu), the largest of the is 120 to 155 days. Winter snowfalls average 89 to native cherries and the only one of commercial value, 203 cm (35 to 80 in), and 45 to 90 days have snow is found throughout the Eastern United States. It is cover of 2.5 cm (1 in) or more. Mean annual potential also known as wild black cherry, rum cherry, and evapotranspiration approximates 430 to 710 mm (17 mountain black cherry. Large, high-quality trees to 28 in), and mean annual water surplus is 100 to suited for furniture wood or veneer are found in large 610 mm (4 to 24 in). January temperatures average numbers in a more restricted commercial range on a maximum of 1” to 6” C (34” to 43” F) and a mini- the Allegheny Plateau of Pennsylvania, New York, mum of -11” to -6” C (12’ to 22” F). July tempera- and West Virginia (36,441. Smaller quantities of tures average a maximum of 27” to 29” C (SO’ to 85” high-quality trees grow in scattered locations along F) and a minimum of 11” to 16” C (52” to 60” F) (42). the southern Appalachian Mountains and the upland areas of the Gulf Coastal Plain. Elsewhere, black cherry is often a small, poorly formed tree of rela- Soils and Topography tively low commercial value, but important to wildlife for its fruit. Throughout its range in eastern North America, black cherry grows well on a wide variety of soils if Habitat summer growing conditions are cool and moist. -

Prunus X Yedoensis Yoshino Cherry1 Edward F
Fact Sheet ST-523 October 1994 Prunus x yedoensis Yoshino Cherry1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Yoshino Cherry grows quickly to 20 feet, has beautiful bark marked with prominent lenticels but is a relatively short-lived tree (Fig. 1). It has upright to horizontal branching, making it ideal for planting along walks and over patios. The white to pink flowers which occur in early spring before the leaves develop are sometimes damaged by late frosts or very windy conditions. This is the tree along with ‘Kwanzan’ Cherry in Washington, DC, which makes such a show each spring. Figure 1. Mature Yoshino Cherry. GENERAL INFORMATION DESCRIPTION Scientific name: Prunus x yedoensis Pronunciation: PROO-nus x yed-oh-EN-sis Height: 35 to 45 feet Common name(s): Yoshino Cherry Spread: 30 to 40 feet Family: Rosaceae Crown uniformity: symmetrical canopy with a USDA hardiness zones: 5B through 8A (Fig. 2) regular (or smooth) outline, and individuals have more Origin: not native to North America or less identical crown forms Uses: Bonsai; wide tree lawns (>6 feet wide); Crown shape: round; vase shape medium-sized tree lawns (4-6 feet wide); Crown density: moderate recommended for buffer strips around parking lots or Growth rate: medium for median strip plantings in the highway; near a deck Texture: medium or patio; shade tree; narrow tree lawns (3-4 feet wide); specimen; sidewalk cutout (tree pit); no proven urban Foliage tolerance Availability: generally available in many areas within Leaf arrangement: alternate (Fig. 3) its hardiness range Leaf type: simple Leaf margin: double serrate; serrate Leaf shape: elliptic (oval); oblong; ovate Leaf venation: banchidodrome; pinnate Leaf type and persistence: deciduous Leaf blade length: 2 to 4 inches 1. -

Prunus Dulcis (Almond) Size/Shape
Prunus dulcis (Almond) "The coming of spring in Lebanon is announced by white and pink Almond tree blossoms appearing everywhere in early March through April from the coast up to an altitude of 1800 meters. The Almond is cultivated in orchards, but trees can also be found growing in the wild, in forests and woodlands, in abandoned orchards, in open shrub lands, between rocks, and on dry limestone slopes. The trees that grow in the wild produce bitter or semi-sweet almond seeds. Their bitterness comes from a compound that turns into the poison cyanide when it comes into contact with water.This economically important tree has a spreading crown and can grow up to eight meters high. Its leaves are elongated, toothed, and egg-shaped, and they grow in clusters from short stubs on the branches." * * Trees of Lebanon, 2014, Salma Nashabe Talhouk, Mariana M. Yazbek, Khaled Sleem, Arbi J. Sarkissian, Mohammad S. Al-Zein, and Sakra Abo Eid Landscape Information ﺷﺠﺮﺓ ﺍﻟﻠﻮﺯ :Arabic Name Pronounciation: PROO-nus DUL-sis Plant Type: Tree Origin: Mediterranean Heat Zones: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Hardiness Zones: 7, 8, 9, 10 Uses: Specimen, Edible, Cut Flowers / Arrangements, Native to Lebanon Size/Shape Growth Rate: Moderate Tree Shape: Vase Canopy Symmetry: Symmetrical Canopy Density: Open Canopy Texture: Fine Height at Maturity: 5 to 8 m Spread at Maturity: 5 to 8 meters Time to Ultimate Height: 10 to 20 Years Plant Image Prunus dulcis (Almond) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: -

RECOMMENDED SMALL TREES for CITY USE (Less Than 30 Feet)
RECOMMENDED SMALL TREES FOR CITY USE (Less than 30 feet) Scientific Name Common Name Comments Amelanchier arborea Serviceberry, shadbush, Juneberry Very early white flowers. Good for pollinators and wildlife. Amorpha fruticosa False indigobush Can form clusters. Legume. Good for pollinators. Asimina triloba Paw-paw Excellent edible fruit. Good for wildlife. Can be hard to establish. Chionanthus virginicus Fringe Tree Cornus mas Cornelian Cherry (Dogwood) Cornus spp. Shrub dogwoods – Gray, Pale, Can form clusters. Very good for wildlife Red-0sier, Alternate, Silky & pollinators. Corylus americana Hazelnut Very good for wildlife. Crataegus pruinosa Frost hawthorn Thorny, attractive white flowers. Good for wildlife. Hamamelis virginiana Witchhazel Good for pollinators Lindera benzoin Spicebush Very good for butterflies. Sweet-smelling aromatic leaves Malus spp. Crabapples – Iowa and Prairie Can get 25’ tall. Beautiful spring flowers. Good for wildlife. Oxydendrum arborum Sourwood Prunus americana Wild or American plum Can form clusters. Very good for wildlife. Can get 20’ tall. Prunus virginiana Chokecherry Can get 30’ tall. Good for wildlife and pollinators. Rhus aromatic Aromatic sumac Attractive to bees and butterflies. Sambucus canadensis Elderberry Edible black berries – good for wildlife and pollinators. Viburnum spp. Arrowwood, nannyberry, Early white flower clusters, very good for blackhaw wildlife. NOTES: • All the above small trees/shrubs prefer moist soil. Some, like the false Indigobush, silky and red-osier dogwoods, spicebush, and elderberry, can tolerate wet soils. None do well on dry sandy or rocky soils. • All prefer at least 3 hours of sun per day, and flower better when they can get 6 hours or more per day. Spicebush can tolerate full shade, but flowers better with 3-6 hours of sun.