Astrazeneca Business Update Presentation PDF 256KB
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Numeric Listing of Manufacturers That Have Signed Rebate Agreements
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements January 1, 2020 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00172 IVAX PHARMACEUTICALS, INC. 1/1/1991 Y 00003 E.R. SQUIBB & SONS, LLC. 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 00004 HOFFMANN-LA ROCHE 1/1/1991 00178 MISSION PHARMACAL COMPANY 1/1/1991 00006 MERCK & CO., INC. 1/1/1991 Y 00182 GOLDLINE LABORATORIES, INC. 1/1/1991 00007 GLAXOSMITHKLINE 1/1/1991 00185 EON LABS, INC. 1/1/1991 Y 00008 WYETH LABORATORIES 1/1/1991 Y 00186 ASTRAZENECA PHARMACEUTICAL 1/1/1991 Y 00009 PHARMACIA AND UPJOHN COMPA 1/1/1991 Y 00187 BAUSCH HEALTH US, LLC. 1/1/1991 Y 00013 PHARMACIA AND UPJOHN COMPA 1/1/1991 Y 00206 WYETH PHARMACEUTICALS LLC 1/1/1991 Y 00015 MEAD JOHNSON AND COMPANY 1/1/1991 Y 00224 KONSYL PHARMACEUTICALS, INC. 1/1/1992 00023 ALLERGAN INC 1/1/1991 00225 B. F. ASCHER AND COMPANY, INC. 1/1/1991 00024 SANOFI-AVENTIS, US LLC 1/1/1991 Y 00228 ACTAVIS ELIZABETH LLC 1/1/1991 Y 00025 GD. SEARLE LLC DIVISION OF PFIZ 1/1/1991 Y 00245 UPSHER-SMITH LABORATORIES, I 1/1/1991 Y 00026 BAYER HEALTHCARE LLC 1/1/1991 Y 00254 PAR PHARMACEUTTICAL INC. 9/28/2018 00029 GLAXOSMITHKLINE 1/1/1991 00258 FOREST LABORATORIES INC 1/1/1991 00032 ABBVIE INC. 1/1/1991 Y 00259 MERZ PHARMACEUTICALS 1/1/1991 00037 MEDA PHARMACEUTICALS, INC. -

Obesity, Diabetes, & Diet
Obesity, Diabetes, & Diet COMBINING EVIDENCE FOR ALL THREE INTO IMPROVED PATIENT CARE Case Study Louis J. Aronne, MD, FACP Weill Cornell Medical College Columbia University College of Physicians and Surgeons Louis J. Aronne, MD, FACP Disclosures !! Research/Grants: Amylin Pharmaceuticals, Inc.; Arena Pharmaceuticals, Inc.; F. Hoffmann-La Roche, Ltd.; Metabolous Pharmaceuticals, Inc.; Norvo Nordisk; Orexigen Therapeutics, Inc.; Pfizer Inc.; TransTech Pharma, Inc. !! Speakers Bureau: None !! Consultant: Allergan, Inc.; Amylin Pharmaceuticals, Inc.; GI Dynamics, Inc.; GlaxoSmithKline Consumer Healthcare, LP; Johnson & Johnson Pharmaceutical Research & Development, LLC; NeuroSearch, Inc.; Novo Nordisk; Orexigen Therapeutics, Inc.; Roche Laboratories, Inc.; VIVUS, Inc.; Wyeth Pharmaceuticals, Inc. !! Stockholder: Cardiometabolic Support Network, LLC !! Other Financial Interest: None !! Advisory Board: Allergan, Inc.; Amylin Pharmaceuticals, Inc.; GI Dynamics, Inc.; GlaxoSmithKline Consumer Healthcare, LP; Johnson & Johnson Pharmaceutical Research & Development, LLC; NeuroSearch, Inc.; Novo Nordisk; Orexigen Therapeutics, Inc.; Roche Laboratories, Inc.; VIVUS, Inc.; Wyeth Pharmaceuticals, Inc. Father A.V. 04May09: 378 lbs, 5’ 11”, BMI = 53 !! Dx w/ DMII in 2004 !! Long history of obesity !! Can’t control his eating, binges !! HbA1c = 8.4% !! FPG = 166 !! TG = 241 !! UA Microalbumin = 973 !! “Can’t tolerate metformin” !! Considering RYGB, but afraid to have surgery DMII = diabetes mellitus type II; Hb = hemoglobin; FPG = fasting plasma glucose; -

In Re Incretin-Based Therapies Products Liability Litigation Transfer Order
Case MDL No. 2452 Document 71 Filed 08/26/13 Page 1 of 4 UNITED STATES JUDICIAL PANEL on MULTIDISTRICT LITIGATION IN RE: INCRETIN MIMETICS PRODUCTS LIABILITY LITIGATION MDL No. 2452 TRANSFER ORDER Before the Panel: Pursuant to 28 U.S.C. § 1407, plaintiffs in two Southern District of California actions move to centralize this litigation, which involves four anti-diabetic medications that plaintiffs contend cause pancreatic cancer, in the Southern District of California. This litigation currently consists of 53 actions pending in seven districts, as listed on Schedule A.1 All responding parties support centralization. Plaintiffs in fifteen Southern District of California actions and a District of Arizona action support plaintiffs’ motion in its entirety. Defendants2 support centralization in the Southern District of California or, alternatively, the District of Colorado or the Western District of Oklahoma. On the basis of the papers filed and hearing session held, we find that these actions involve common questions of fact, and that centralization of all actions in the Southern District of California will serve the convenience of the parties and witnesses and promote the just and efficient conduct of this litigation. Plaintiffs in all actions allege that the use of one or more of four anti-diabetic incretin- based medications – Janumet (sitagliptin combined with metformin), Januvia (sitagliptin), Byetta (exenatide) and Victoza (liraglutide) – caused them or their decedent to develop pancreatic cancer. Centralization will eliminate duplicative discovery; prevent inconsistent pretrial rulings (particularly on such matters as Daubert rulings); and conserve the resources of the parties, their counsel, and the judiciary. We are “typically hesitant to centralize litigation against multiple, competing defendants which marketed, manufactured and sold similar products.” In re Yellow Brass Plumbing Component Prods. -

Curriculum Vitae
David C. Klonoff MD, FACP, FRCP (Edin), Fellow AIMBE Page 1 CURRICULUM VITAE DAVID CHARLES KLONOFF, M.D., FACP, FRCP (Edin), FELLOW AIMBE Medical Director, Diabetes Research Institute, Mills-Peninsula Medical Center 100 South San Mateo Drive, Room 5147, San Mateo, California 94401 Phone 650-696-4260 / Fax 650-696-4269 [email protected] SUMMARY David C. Klonoff, M.D. is an endocrinologist specializing in the development and use of diabetes technology. He is Medical Director of the Dorothy L. and James E. Frank Diabetes Research Institute of Mills-Peninsula Medical Center in San Mateo, California and a Clinical Professor of Medicine at UCSF. Dr. Klonoff received the American Diabetes Association’s 2019 Outstanding Physician Clinician Award. He received an FDA Director’s Special Citation Award in 2010 for outstanding contributions related to diabetes technology. In 2012 Dr. Klonoff was elected as a Fellow of the American Institute of Medical and Biological Engineering (AIMBE) and cited as among the top 2% of the world’s bioengineers for his engineering work in diabetes technology. He received the 2012 Gold Medal Oration and Distinguished Scientist Award from the Dr. Mohan’s Diabetes Specialities Centre and Madras Diabetes Research Foundation of Chennai, India. Dr. Klonoff was invited to speak to the US Congressional Diabetes Caucus in 2017, participate in the White House Health and Cybersecurity Roundtable in 2015, and speak at the European Parliament in 2010. He is the Founding Editor- in-Chief of Journal of Diabetes Science and Technology. He has authored over 270 publications in PubMed journals including four of the first ten articles on diabetes device cybersecurity. -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Amylin Pharmaceuticals and Eli Lilly Provide Context for FDA Alert for BYETTA
Amylin Pharmaceuticals and Eli Lilly Provide Context for FDA Alert for BYETTA SAN DIEGO and INDIANAPOLIS, Aug 26, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Amylin Pharmaceuticals, Inc. (Nasdaq: AMLN) and Eli Lilly and Company (NYSE: LLY) in a conference call today provided context and additional information regarding the August 18, 2008 U.S. Food and Drug Administration (FDA) update to a prior alert for BYETTA® (exenatide) injection referencing pancreatitis. The companies were aware of the pancreatitis cases referenced in the alert, as well as others, and previously reported these cases to the FDA. The complete conference call replay will be available through Amylin's and Lilly's corporate websites after the call. Since 2006, the U.S. prescribing information for BYETTA has included information about pancreatitis. A recent study has also shown that patients with type 2 diabetes were at nearly three times the risk of developing pancreatitis than those without diabetes.(1) While a definite causal relationship between BYETTA and pancreatitis has not been proved, to better understand the suspected relationship, Amylin and Lilly continue to pursue a comprehensive drug safety program that includes extensive internal and external review of individual cases, and clinical and epidemiologic studies. "At Amylin and Lilly, patient safety is our foremost concern. We are committed to continuing to work closely with the FDA to ensure that physicians and patients are provided with accurate information about any potential risks associated with the use of our products," said Orville G. Kolterman, Senior Vice President, Research and Development at Amylin. "It is important to understand that pancreatitis, an inflammatory condition of the pancreas, is a rare event. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 55513 AMGEN USA 00074 ABBOTT LABORATORIES 58406 AMGEN/IMMUNEX 68817 ABRAXIS BIOSCIENCE, LLC 53746 AMNEAL PHARMACEUTICALS 16729 ACCORD HEALTHCARE INCORPORATED 65162 AMNEAL PHARMACEUTICALS LLC 42192 ACELLA PHARMACEUTICALS, LLC 69238 AMNEAL PHARMACEUTICALS, LLC 10144 ACORDA THERAPEUTICS, INC. 53150 AMNEAL-AGILA, LLC 00472 ACTAVIS 00548 AMPHASTAR PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 69918 AMRING PHARMACEUTICALS, INC. 45963 ACTAVIS INC. 66780 AMYLIN PHARMACEUTICALS, INC. 46987 ACTAVIS KADIAN LLC 55724 ANACOR PHARMACEUTICALS 49687 ACTAVIS KADIAN LLC 10370 ANCHEN PHARMACEUTICALS, INC. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 43595 ANGELINI PHARMA, INC. 61874 ACTAVIS PHARMA, INC. 62559 ANIP ACQUISITION COMPANY 67767 ACTAVIS SOUTH ATLANTIC 54436 ANTARES PHARMA, INC. 66215 ACTELION PHARMACEUTICALS U.S., INC. 52609 APO-PHARMA USA, INC. 52244 ACTIENT PHARMACEUTICALS 60505 APOTEX CORP. 75989 ACTON PHARMACEUTICALS 63323 APP PHARMACEUTICALS, LLC. 69547 ADAPT PHARMA INC. 43485 APRECIA PHARMACEUTICALS COMPANY 76431 AEGERION PHARMACEUTICALS, INC. 42865 APTALIS PHARMA US, INC 50102 AFAXYS, INC. 58914 APTALIS PHARMA US, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 13310 AR SCIENTIFIC, INC. 27241 AJANTA PHARMA LIMITED 08221 ARBOR PHARM IRELAND LIMITED 17478 AKORN INC 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 24090 AKRIMAX PHARMACEUTICALS LLC 24338 ARBOR PHARMACEUTICALS, INC. 68220 ALAVEN PHARMACEUTICAL, LLC 59923 AREVA PHARMACEUTICALS 00065 ALCON LABORATORIES, INC. 76189 ARIAD PHARMACEUTICALS, INC. 00998 ALCON LABORATORIES, INC. 24486 ARISTOS PHARMACEUTICALS, INC. -

Preliminary Healthcare Agenda 01.03X
29th Annual J.P. Morgan Healthcare Conference January 10 - 13, 2011 Westin St. Francis Hotel, San Francisco, CA Preliminary Conference Agenda SUNDAY, JANUARY 9 - Registration in Tower Salon A - 3 to 9 PM MONDAY, JANUARY 10 - Registration in Tower Salon A - 6:45 AM, Breakfast in Italian Foyer Grand Ballroom Colonial Room California West California East Elizabethan A/B Elizabethan C/D Alexandra's Breakout: Borgia Room Breakout: Georgian Room Breakout: Olympic Room Breakout: Yorkshire Room Breakout: Sussex Room Private Company Track Not-for-Profit Track 7:30 AM Opening Remarks: Doug Braunstein - Chief Financial Officer, JPMorgan Chase & Co., Grand Ballroom Astra Tech 8:00 AM Celgene Corporation Kinetic Concepts, Inc Alkermes, Inc. Biocon Limited Catalent (private company) 8:30 AM Express Scripts Inc. Agilent Technologies Inc. Beckman Coulter Inc. Bio-Rad Laboratories, Inc. Quality Systems Axcan Intermediate Holdings 9:00 AM Roche Holding AG Zimmer Holdings, Inc. Genoptix, Inc. ImmunoGen, Inc Health Net Inc. Merrimack Pharmaceuticals Inc. Vertex Pharmaceuticals Allscripts Healthcare Solutions, 9:30 AM Medicis Pharmaceutical Corp. Lonza Group Ltd Henry Schein Inc. Surgical Care Affiliates Incorporated Inc. 10:00 AM Medtronic, Inc. WellPoint, Inc. Onyx Pharmaceuticals Inc. Sigma-Aldrich Corporation Align Technology Inc.* Symphogen 10:30 AM Room Not Available Medco Health Solutions, Inc. Smith & Nephew plc* Medivation, Inc. Lexicon Pharmaceuticals, Inc. Zeltiq Aesthetics 11:00 AM Room Not Available Merck KGaA Perrigo Company Healthways Incorporated BioMimetic Therapeutics, Inc. Penumbra, Inc. 11:30 AM Room Not Available Dendreon Corporation Gen-Probe Inc. Select Medical Corporation ArthroCare Corporation PTC Therapeutics, Inc. 12:00 PM Luncheon & Keynote: Nancy-Ann DeParle - Counselor to the President and Director of the White House Office of Health Reform, Grand Ballroom Endo Pharmaceuticals Holdings 1:30 PM Room Not Available Amylin Pharmaceuticals Inc. -

Brand Name: Bydureon Generic Name: Exenatide Extended-Release
Brand Name: Bydureon Generic Name: Exenatide extended-release (ER) Manufacturer: Amylin Pharmaceuticals Drug Class 1,2,3,4,5: Antidiabetic; glucagon-like peptide-1 (GLP-1) receptor agonist Uses: Labeled Uses1,2,3,4, 5: Treatment of type 2 diabetes mellitus (noninsulin dependent DM) to improve glycemic control. Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Exenatide extended-release is not recommended as first-line therapy. Concomitant use with insulin is not recommended. Unlabeled Uses1: No current unlabeled use Mechanism of Action:1,2,3,4,: Incretins, such as glucagon-like peptide-1 (GLP-1), enhance glucose-dependent insulin secretion and exhibit other antihyperglycemic actions following their release into the circulation from the gut. Exenatide extended-release is a GLP-1 receptor agonist that enhances glucose-dependent insulin secretion by the pancreatic beta-cell, suppresses inappropriately elevated glucagon secretion, slows gastric emptying, reduces food intake, and promotes β-cell proliferation. Exenatide acutely improves glycemic control by reducing fasting and postprandial glucose concentrations in patients with type 2 diabetes. Exenatide leads to a release of insulin only in the presence of elevated glucose concentrations. Insulin secretion subsides as euglycemia occurs. First-phase insulin response (release of insulin within 10 minutes following a glucose load) is lost in patients with type 2 diabetes. Loss of first-phase response is a beta cell defect. Exenatide restores first-phase insulin response to an IV bolus of glucose. Exenatide moderates glucagon secretion and lowers serum glucagon concentrations during periods of hyperglycemia. Lower glucagon concentrations lead to decreased hepatic glucose output and decreased insulin demand. -

AMYLIN PHARMACEUTICALS, INC. PATIENT ASSISTANCE APPLICATION Phone Toll Free: 1-800-330-7647
AMYLIN PHARMACEUTICALS, INC. PATIENT ASSISTANCE APPLICATION Phone Toll Free: 1-800-330-7647 ____BYETTA (exenatide) injection New Application Renewal Patient Information: Mr. Mrs. Ms. Patient Name _________________________ Mailing Address ______ City State Zip Code Day Phone Evening Phone Date of Birth ________ Social Security # Do you have Type 2 Diabetes? Yes No Marital Status: Single Married Divorced Separated Widowed Are you a U.S. citizen? Yes No (If no, please provide proof of US residency, such as copy of your Driver’s License, Green Card or other government-issued ID that identifies your address) Are you employed? Yes ( Full-time or Part-time) No Self-employed Employer Name ________________________________________________________ Employer Address ______________________________________________________ Household Size (number of persons dependent upon total household income): Physician Information: Physician Name Address City State Zip Code Phone Fax Insurance Information: Are you a participant in any of the following? (Check all that apply): Medicare Part A Medicare Part B Medicare Part C (Medicare Advantage) Medicare Part D (Medicare prescription drug plan) Medicaid TriCare/CHAMPUS Veterans Administration Indian Health Services Public Health Service Any other Federal or State healthcare program, please list Page 1 of 2 Do you have private health insurance? Yes No If yes, name of Primary Insurer: _____________________________________ ID # _______________________ Phone __________________ Name of Secondary Insurer: ________________________________________ ID # _______________________ Phone __________________ Have you applied for Medicaid? Yes No If yes, date you applied _____________ Were you approved? Yes No Financial Information (Documentation of income required): Household Income: Please identify your household’s Adjusted Gross Income as it appeared on the most recent year’s federal tax return (IRS Form 1040): $ . -

11.Absfrontpageduality ADA 13.Indd
PRESENTED AND PUBLISHED ABSTRACTS DISCLOSURE INFORMATION All presenters/authors are required to disclose to the program audience any fi nancial relationships related to the subject matter of this program. The intent of this disclosure is not to prevent a presenter or author from being involved in the activity, but rather to provide participants with information on which they may make their own judgments. AUTHOR DISCLOSURE A829 AUTHOR DISCLOSURE A830 ABSTRACT AUTHOR DISCLOSURE INFORMATION AUTHOR RELATIONSHIP/COMPANY AUTHOR RELATIONSHIP/COMPANY Aakhus, Svend ...................................... Disclosed no confl ict of interest. Adhami, Anwar .................................... Disclosed no confl ict of interest. Aalgur, Fusun ........................................Disclosed no confl ict of interest. Adhami, Mitra ......................................Disclosed no confl ict of interest. Aamidor, Sarah E. .................................Disclosed no confl ict of interest. Adhya, Sumona .................................... Employee: Medtronic Abayomi, Julie ..................................... Disclosed no confl ict of interest. Adkins, Laura ........................................ Disclosed no confl ict of interest. Abbas, Afroze .......................................Disclosed no confl ict of interest. Adler, Benjamin ....................................Disclosed no confl ict of interest. Abbasi, Fahim ....................................... Disclosed no confl ict of interest. Adler, Steffen ....................................... Consultant: -
Crystal Reports Activex Designer
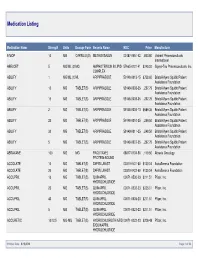
Medication Listing Medication Name Strength Units Dosage Form Generic Name NDC Price Manufacturer 8-MOP 10 MG CAPSULE(S) METHOXSALEN 00187-0651-42 1,843.80 Valeant Pharmaceuticals International ABELCET 5 MG/ML (20MG AMPHOTERICIN B LIPID 57665-0101-41 $240.00 Sigma-Tau Pharmaceuticals, Inc. COMPLEX ABILIFY 1 MG/ML (150ML ARIPIPRAZOLE 59148-0013-15 $758.60 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 10 MG TABLET(S) ARIPIPRAZOLE 59148-0008-35 2,297.75 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 15 MG TABLET(S) ARIPIPRAZOLE 59148-0009-35 2,297.75 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 2 MG TABLET(S) ARIPIPRAZOLE 59148-0006-13 $689.36 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 20 MG TABLET(S) ARIPIPRAZOLE 59148-0010-35 3,249.50 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 30 MG TABLET(S) ARIPIPRAZOLE 59148-0011-35 3,249.50 Bristol-Myers Squibb Patient Assistance Foundation ABILIFY 5 MG TABLET(S) ARIPIPRAZOLE 59148-0007-35 2,297.75 Bristol-Myers Squibb Patient Assistance Foundation ABRAXANE 100 MG MG PACLITAXEL 68817-0134-50 1,119.60 Abraxis Oncology PROTEIN-BOUND ACCOLATE 10 MG TABLET(S) ZAFIRLUKAST 00310-0401-60 $130.04 AstraZeneca Foundation ACCOLATE 20 MG TABLET(S) ZAFIRLUKAST 00310-0402-60 $130.04 AstraZeneca Foundation ACCUPRIL 10 MG TABLET(S) QUINAPRIL 00071-0530-23 $211.51 Pfizer, Inc. HYDROCHLORIDE ACCUPRIL 20 MG TABLET(S) QUINAPRIL 00071-0532-23 $235.01 Pfizer, Inc. HYDROCHLORIDE ACCUPRIL 40 MG TABLET(S) QUINAPRIL 00071-0535-23 $211.51 Pfizer, Inc. HYDROCHLORIDE ACCUPRIL 5 MG TABLET(S) QUINAPRIL 00071-0527-23 $211.51 Pfizer, Inc.