Crystal Reports Activex Designer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -
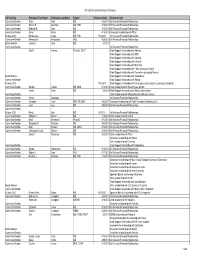
CB2013 Committee and Board Disclosureauditlist 9.10.13
2013-2014 Conflict of Interest Disclosure AST Activity Participant First Name Participant Last Name Degree DisclosureDate Disclsoure Item Committee Member Reza Abdi MD 4/22/2013 No Relevant Financial Relationships Committee Member Sameh R. Abul-Ezz MD, PhD 4/16/2013 No Relevant Financial Relationships Committee Member Deborah B. Adey MD 4/15/2013 No Relevant Financial Relationships Committee Member Enver Akalin MD 4/16/2013 Consultant relationship with Pfizer Fellows 2013 Maria-Luisa Alegre MD, PhD 7/12/2013 No Relevant Financial Relationships Committee Member Alessandrini Alessandro PhD 4/29/2013 No Relevant Financial Relationships Board Member James S. Allan MD 5/3/2013 Committee Member No Relevant Financial Relationships Rita R. Alloway PharmD, FCCP Grant Support relationship with Astellas Grant Support relationship with BMS Grant Support relationship with Novartis Grant Support relationship with Lifecycle Grant Support relationship with Millenium Grant Support relationship with Pfizer (previously Wyeth) Grant Support relationship with Genentech (previously Roche) Board Member Grant Support relationship with Viropharma Committee Member Grant Support relationship with Alexion Fellows 2013 7/18/2013 Grant Support relationship with Sanofi (previously Genzyme, previously Sangstat) Committee Member Sandra Amaral MD, MHS 6/18/2013 Other relationship with Bristol Myers-Squibb Hatem Amer MD 5/6/2013 Grant Support relationship with Abbott Laborartories. Committee Member Other relationship with Massacheussetts Medical Society. Committee Member William Applegate No Relevant Financial Relationships Committee Member Alexander Aussi BSN, RN, MBA 4/22/2013 Consultant relationship with Total Transplant Advantage, LLC Committee Member Jamil Azzi MD 4/30/2013 No Relevant Financial Relationships Committee Member Fellows 2013 Mark L. -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

Federal Register/Vol. 84, No. 232/Tuesday, December 3, 2019
Federal Register / Vol. 84, No. 232 / Tuesday, December 3, 2019 / Notices 66191 the Assistant Attorney General, patterns, devices, manufacturing filed with and accepted, subject to final developed the HSR Rules and the processes, or customer names. approval, by the Commission, has been corresponding Notification and Report placed on the public record for a period Form. Heather Hippsley, of thirty (30) days. The following On September 11, 2019, the Deputy General Counsel. Analysis to Aid Public Comment Commission sought comment on the [FR Doc. 2019–26075 Filed 12–2–19; 8:45 am] describes the terms of the consent reporting requirements associated with BILLING CODE 6750–01–P agreement and the allegations in the the HSR Rules and corresponding complaint. An electronic copy of the Notification and Report Form. 84 FR full text of the consent agreement 47951. No relevant comments were FEDERAL TRADE COMMISSION package can be obtained from the FTC received. Pursuant to the OMB [File No. 191 0061] Home Page (for November 15, 2019), on regulations, 5 CFR part 1320, that the World Wide Web, at https:// implement the PRA, 44 U.S.C. 3501 et Bristol-Myers Squibb Company and www.ftc.gov/news-events/commission- seq., the FTC is providing this second Celgene Corporation; Analysis of actions. opportunity for public comment while Agreement Containing Consent Orders You can file a comment online or on seeking OMB approval to renew the pre- To Aid Public Comment paper. For the Commission to consider existing clearance for those information your comment, we must receive it on or collection requirements. -

Other Statutory Disclosures Continued
Other statutory disclosures continued Strategic reportGroup companies Governance & remuneration Financial statements In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the country of incorporation and effective percentage of equity owned, as at 31 December 2015 are disclosed below. Unless otherwise stated the share capital disclosed comprises ordinary shares which are indirectly held by GlaxoSmithKline plc. All subsidiary companies are resident for tax purposes in their country of incorporation unless otherwise stated. Country of Effective % % Held by Name incorporation Ownership Security Class of Share Wholly owned subsidiaries 1506369 Alberta ULC Canada 100 Common 100 Action Potential Venture Capital Limited England & Wales 100 Ordinary 100 Adechsa GmbH Switzerland 100 Ordinary 100 Affymax Research Institute United States 100 Common 100 Alenfarma – Especialidades Farmaceuticas, Limitada (iv) Portugal 100 Ordinary Quota 100 Allen & Hanburys Limited (iv) England & Wales 100 Ordinary 100 Allen & Hanburys Pharmaceutical Nigeria Limited Nigeria 100 Ordinary 100 Allen Farmaceutica, S.A. Spain 100 Ordinary 100 Allen Pharmazeutika Gesellschaft m.b.H. Austria 100 Ordinary 100 Aners S.A (iv) Argentina 100 Non-endorsable Nominative Ordinary 100 Barrier Therapeutics, Inc. United States 100 Common 100 Beecham Group p l c England & Wales 100 20p Shares 'A'; 5p Shares B 100 Beecham Pharmaceuticals (Pte) Limited Singapore 100 Ordinary 100 Beecham Pharmaceuticals S.A (iv) (vi) Ecuador 100 Nominative 100 Beecham Portuguesa-Produtos Farmaceuticos e Quimicos, Lda Portugal 100 Ordinary Quota 100 Beecham S.A. (iv) Belgium 100 Ordinary 100 Biddle Sawyer Limited India 100 Equity 100 Biovesta Ilaçlari Ltd. Sti. Turkey 100 Nominative 100 Burroughs Wellcome & Co (Australia) Pty Limited (iv) (vi) Australia 100 Ordinary 100 Burroughs Wellcome & Co (Bangladesh) Limited Bangladesh 100 Ordinary 100 Burroughs Wellcome International Limited England & Wales 100 Ordinary 100 Caribbean Chemical Company, Ltd. -

Numeric Listing of Manufacturers That Have Signed Rebate Agreements
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements January 1, 2020 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00172 IVAX PHARMACEUTICALS, INC. 1/1/1991 Y 00003 E.R. SQUIBB & SONS, LLC. 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 00004 HOFFMANN-LA ROCHE 1/1/1991 00178 MISSION PHARMACAL COMPANY 1/1/1991 00006 MERCK & CO., INC. 1/1/1991 Y 00182 GOLDLINE LABORATORIES, INC. 1/1/1991 00007 GLAXOSMITHKLINE 1/1/1991 00185 EON LABS, INC. 1/1/1991 Y 00008 WYETH LABORATORIES 1/1/1991 Y 00186 ASTRAZENECA PHARMACEUTICAL 1/1/1991 Y 00009 PHARMACIA AND UPJOHN COMPA 1/1/1991 Y 00187 BAUSCH HEALTH US, LLC. 1/1/1991 Y 00013 PHARMACIA AND UPJOHN COMPA 1/1/1991 Y 00206 WYETH PHARMACEUTICALS LLC 1/1/1991 Y 00015 MEAD JOHNSON AND COMPANY 1/1/1991 Y 00224 KONSYL PHARMACEUTICALS, INC. 1/1/1992 00023 ALLERGAN INC 1/1/1991 00225 B. F. ASCHER AND COMPANY, INC. 1/1/1991 00024 SANOFI-AVENTIS, US LLC 1/1/1991 Y 00228 ACTAVIS ELIZABETH LLC 1/1/1991 Y 00025 GD. SEARLE LLC DIVISION OF PFIZ 1/1/1991 Y 00245 UPSHER-SMITH LABORATORIES, I 1/1/1991 Y 00026 BAYER HEALTHCARE LLC 1/1/1991 Y 00254 PAR PHARMACEUTTICAL INC. 9/28/2018 00029 GLAXOSMITHKLINE 1/1/1991 00258 FOREST LABORATORIES INC 1/1/1991 00032 ABBVIE INC. 1/1/1991 Y 00259 MERZ PHARMACEUTICALS 1/1/1991 00037 MEDA PHARMACEUTICALS, INC. -

Shire Acquisition of Viropharma - Strategic Move to Strengthen Shire’S Rare Disease Business - Augments Already Strong Growth Prospects
Shire acquisition of ViroPharma - Strategic move to strengthen Shire’s Rare Disease business - Augments already strong growth prospects Flemming Ornskov, MD Chief Executive Officer Graham Hetherington Chief Financial Officer Our purpose We enable people with life-altering conditions to lead better lives. CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS Statements included in this communication that are not historical facts are forward-looking statements. Forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: •Shire’s proposed acquisition of ViroPharma may not be consummated due to the occurrence of an event, change or other circumstances that gives rise to the termination of the merger agreement; •a governmental or regulatory approval required for the proposed acquisition of ViroPharma may not be obtained, or may be obtained subject to conditions that are not anticipated, or another condition to the closing of the proposed acquisition may not be satisfied; •ViroPharma may be unable to retain and hire key personnel and/or maintain its relationships with customers, suppliers and other business partners pending the consummation of the proposed acquisition by Shire, or ViroPharma’s business may be disrupted by the proposed acquisition, including increased costs and diversion of management time and resources; •difficulties in integrating ViroPharma into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; and risks and uncertainties detailed from time to time in Shire’s or ViroPharma’s filings with the U.S. -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -

Obesity, Diabetes, & Diet
Obesity, Diabetes, & Diet COMBINING EVIDENCE FOR ALL THREE INTO IMPROVED PATIENT CARE Case Study Louis J. Aronne, MD, FACP Weill Cornell Medical College Columbia University College of Physicians and Surgeons Louis J. Aronne, MD, FACP Disclosures !! Research/Grants: Amylin Pharmaceuticals, Inc.; Arena Pharmaceuticals, Inc.; F. Hoffmann-La Roche, Ltd.; Metabolous Pharmaceuticals, Inc.; Norvo Nordisk; Orexigen Therapeutics, Inc.; Pfizer Inc.; TransTech Pharma, Inc. !! Speakers Bureau: None !! Consultant: Allergan, Inc.; Amylin Pharmaceuticals, Inc.; GI Dynamics, Inc.; GlaxoSmithKline Consumer Healthcare, LP; Johnson & Johnson Pharmaceutical Research & Development, LLC; NeuroSearch, Inc.; Novo Nordisk; Orexigen Therapeutics, Inc.; Roche Laboratories, Inc.; VIVUS, Inc.; Wyeth Pharmaceuticals, Inc. !! Stockholder: Cardiometabolic Support Network, LLC !! Other Financial Interest: None !! Advisory Board: Allergan, Inc.; Amylin Pharmaceuticals, Inc.; GI Dynamics, Inc.; GlaxoSmithKline Consumer Healthcare, LP; Johnson & Johnson Pharmaceutical Research & Development, LLC; NeuroSearch, Inc.; Novo Nordisk; Orexigen Therapeutics, Inc.; Roche Laboratories, Inc.; VIVUS, Inc.; Wyeth Pharmaceuticals, Inc. Father A.V. 04May09: 378 lbs, 5’ 11”, BMI = 53 !! Dx w/ DMII in 2004 !! Long history of obesity !! Can’t control his eating, binges !! HbA1c = 8.4% !! FPG = 166 !! TG = 241 !! UA Microalbumin = 973 !! “Can’t tolerate metformin” !! Considering RYGB, but afraid to have surgery DMII = diabetes mellitus type II; Hb = hemoglobin; FPG = fasting plasma glucose; -

Package Insert Has Been Approved by the U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION **Doses up to 1,000 U every 3 to 4 days may be considered based on These highlights do not include all the information needed to use individual patient response. ® CINRYZE safely and effectively. See full prescribing information for ---------------------- DOSAGE FORMS AND STRENGTHS -------------------- CINRYZE. Approximately 500 Units (lyophilized) in an 8 mL vial. (3) CINRYZE (C1 Esterase Inhibitor [Human]) ---------------------------- CONTRAINDICATIONS ------------------------------- For Intravenous Use, Freeze-Dried Powder for Reconstitution Patients who have manifested life-threatening immediate hypersensitivity Initial U.S. Approval: 2008. reactions, including anaphylaxis, to the product (4). --------------------------- RECENT MAJOR CHANGES -------------------------- ----------------------------- WARNINGS/PRECAUTIONS ------------------------ • Indications and Usage (1) 06/2018 • Hypersensitivity reactions may occur. Have epinephrine immediately • Dosage and Administration (2.1) 06/2018 available for treatment of acute severe hypersensitivity reaction (5.1) • --------------------------- INDICATIONS AND USAGE --------------------------- Serious arterial and venous thromboembolic (TE) events have been CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against reported at the recommended dose of C1 Esterase Inhibitor (Human) angioedema attacks in adults, adolescents and pediatric patients (6 years of products, including CINRYZE, following administration in patients with age and older) -

In Re Incretin-Based Therapies Products Liability Litigation Transfer Order
Case MDL No. 2452 Document 71 Filed 08/26/13 Page 1 of 4 UNITED STATES JUDICIAL PANEL on MULTIDISTRICT LITIGATION IN RE: INCRETIN MIMETICS PRODUCTS LIABILITY LITIGATION MDL No. 2452 TRANSFER ORDER Before the Panel: Pursuant to 28 U.S.C. § 1407, plaintiffs in two Southern District of California actions move to centralize this litigation, which involves four anti-diabetic medications that plaintiffs contend cause pancreatic cancer, in the Southern District of California. This litigation currently consists of 53 actions pending in seven districts, as listed on Schedule A.1 All responding parties support centralization. Plaintiffs in fifteen Southern District of California actions and a District of Arizona action support plaintiffs’ motion in its entirety. Defendants2 support centralization in the Southern District of California or, alternatively, the District of Colorado or the Western District of Oklahoma. On the basis of the papers filed and hearing session held, we find that these actions involve common questions of fact, and that centralization of all actions in the Southern District of California will serve the convenience of the parties and witnesses and promote the just and efficient conduct of this litigation. Plaintiffs in all actions allege that the use of one or more of four anti-diabetic incretin- based medications – Janumet (sitagliptin combined with metformin), Januvia (sitagliptin), Byetta (exenatide) and Victoza (liraglutide) – caused them or their decedent to develop pancreatic cancer. Centralization will eliminate duplicative discovery; prevent inconsistent pretrial rulings (particularly on such matters as Daubert rulings); and conserve the resources of the parties, their counsel, and the judiciary. We are “typically hesitant to centralize litigation against multiple, competing defendants which marketed, manufactured and sold similar products.” In re Yellow Brass Plumbing Component Prods. -

Curriculum Vitae
David C. Klonoff MD, FACP, FRCP (Edin), Fellow AIMBE Page 1 CURRICULUM VITAE DAVID CHARLES KLONOFF, M.D., FACP, FRCP (Edin), FELLOW AIMBE Medical Director, Diabetes Research Institute, Mills-Peninsula Medical Center 100 South San Mateo Drive, Room 5147, San Mateo, California 94401 Phone 650-696-4260 / Fax 650-696-4269 [email protected] SUMMARY David C. Klonoff, M.D. is an endocrinologist specializing in the development and use of diabetes technology. He is Medical Director of the Dorothy L. and James E. Frank Diabetes Research Institute of Mills-Peninsula Medical Center in San Mateo, California and a Clinical Professor of Medicine at UCSF. Dr. Klonoff received the American Diabetes Association’s 2019 Outstanding Physician Clinician Award. He received an FDA Director’s Special Citation Award in 2010 for outstanding contributions related to diabetes technology. In 2012 Dr. Klonoff was elected as a Fellow of the American Institute of Medical and Biological Engineering (AIMBE) and cited as among the top 2% of the world’s bioengineers for his engineering work in diabetes technology. He received the 2012 Gold Medal Oration and Distinguished Scientist Award from the Dr. Mohan’s Diabetes Specialities Centre and Madras Diabetes Research Foundation of Chennai, India. Dr. Klonoff was invited to speak to the US Congressional Diabetes Caucus in 2017, participate in the White House Health and Cybersecurity Roundtable in 2015, and speak at the European Parliament in 2010. He is the Founding Editor- in-Chief of Journal of Diabetes Science and Technology. He has authored over 270 publications in PubMed journals including four of the first ten articles on diabetes device cybersecurity.