Non-Merger Civil Enforcement: an Overview of Recent DOJ and FTC Federal Court Litigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -
CB2013 Committee and Board Disclosureauditlist 9.10.13
2013-2014 Conflict of Interest Disclosure AST Activity Participant First Name Participant Last Name Degree DisclosureDate Disclsoure Item Committee Member Reza Abdi MD 4/22/2013 No Relevant Financial Relationships Committee Member Sameh R. Abul-Ezz MD, PhD 4/16/2013 No Relevant Financial Relationships Committee Member Deborah B. Adey MD 4/15/2013 No Relevant Financial Relationships Committee Member Enver Akalin MD 4/16/2013 Consultant relationship with Pfizer Fellows 2013 Maria-Luisa Alegre MD, PhD 7/12/2013 No Relevant Financial Relationships Committee Member Alessandrini Alessandro PhD 4/29/2013 No Relevant Financial Relationships Board Member James S. Allan MD 5/3/2013 Committee Member No Relevant Financial Relationships Rita R. Alloway PharmD, FCCP Grant Support relationship with Astellas Grant Support relationship with BMS Grant Support relationship with Novartis Grant Support relationship with Lifecycle Grant Support relationship with Millenium Grant Support relationship with Pfizer (previously Wyeth) Grant Support relationship with Genentech (previously Roche) Board Member Grant Support relationship with Viropharma Committee Member Grant Support relationship with Alexion Fellows 2013 7/18/2013 Grant Support relationship with Sanofi (previously Genzyme, previously Sangstat) Committee Member Sandra Amaral MD, MHS 6/18/2013 Other relationship with Bristol Myers-Squibb Hatem Amer MD 5/6/2013 Grant Support relationship with Abbott Laborartories. Committee Member Other relationship with Massacheussetts Medical Society. Committee Member William Applegate No Relevant Financial Relationships Committee Member Alexander Aussi BSN, RN, MBA 4/22/2013 Consultant relationship with Total Transplant Advantage, LLC Committee Member Jamil Azzi MD 4/30/2013 No Relevant Financial Relationships Committee Member Fellows 2013 Mark L. -

XCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) (X) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 ( ) TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 0-19034 REGENERON PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) New York 13-3444607 (State or other jurisdiction of (I.R.S. Employer Identification No) incorporation or organization) 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 (Address of principal executive offices) (Zip code) (914) 347-7000 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None (Title of Class) Securities registered pursuant to Section 12(g) of the Act: Common Stock - par value $.001 per share (Title of Class) Preferred Share Purchase Rights expiring October 18, 2006 (Title of Class) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes /X/ No Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (ss. 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -

Virginia: in the Circuit Court of Pittsylvania County
VIRGINIA: IN THE CIRCUIT COURT OF PITTSYLVANIA COUNTY PITTSYLVANIA COUNTY, Plaintiff, v. PURDUE PHARMA, L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; RHODES PHARMACEUTICALS, L.P.; ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC.; MALLINCKRODT PLC; MALLINCKRODT LLC; ENDO HEALTH SOLUTIONS, INC; ENDO PHARMACEUTICALS, INC.; PAR Case No. CL18 - __________ PHARMACEUTICAL COMPANIES, INC.; PAR PHARMACEUTICAL, INC.; TEVA Jury Trial Demanded PHARMACEUTICALS USA, INC.; CEPHALON, INC.; BARR LABORATORIES, INC.; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC.; WATSON LABORATORIES, INC.; ALLERGAN PLC; ACTAVIS PHARMA, INC.; ACTAVIS, LLC; INSYS THERAPEUTICS, INC.; KVK-TECH, INC.; AMNEAL PHARMACEUTICALS LLC; IMPAX LABORATORIES, LLC; AMNEAL PHARMACEUTICALS, INC.; AMNEAL PHARMACEUTICALS OF NEW YORK, LLC; MYLAN PHARMACEUTICALS, INC.; MCKESSON CORPORATION; MCKESSON MEDICAL-SURGICAL INC.; CARDINAL HEALTH, INC.; AMERISOURCEBERGEN DRUG CORPORATION; HENRY SCHEIN, INC.; GENERAL INJECTABLES & VACCINES, INC.; INSOURCE, INC.; CVS HEALTH CORPORATION; CVS PHARMACY, INC.; CVS TN DISTRIBUTION, L.L.C.; WALGREENS BOOTS ALLIANCE, INC.; WALGREEN CO.; EXPRESS SCRIPTS HOLDING COMPANY; EXPRESS SCRIPTS, INC; CAREMARK RX, L.L.C.; CAREMARKPCS HEALTH, L.L.C.; CAREMARK, L.L.C.; UNITEDHEALTH GROUP INCORPORATED; OPTUM, INC.; OPTUMRX, INC.; and DOES 1-100, Defendants. PLAINTIFF’S ORIGINAL COMPLAINT Plaintiff, Pittsylvania County, Virginia, by and through the undersigned attorneys, (hereinafter “Plaintiff,” “Pittsylvania -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

Federal Register/Vol. 84, No. 232/Tuesday, December 3, 2019
Federal Register / Vol. 84, No. 232 / Tuesday, December 3, 2019 / Notices 66191 the Assistant Attorney General, patterns, devices, manufacturing filed with and accepted, subject to final developed the HSR Rules and the processes, or customer names. approval, by the Commission, has been corresponding Notification and Report placed on the public record for a period Form. Heather Hippsley, of thirty (30) days. The following On September 11, 2019, the Deputy General Counsel. Analysis to Aid Public Comment Commission sought comment on the [FR Doc. 2019–26075 Filed 12–2–19; 8:45 am] describes the terms of the consent reporting requirements associated with BILLING CODE 6750–01–P agreement and the allegations in the the HSR Rules and corresponding complaint. An electronic copy of the Notification and Report Form. 84 FR full text of the consent agreement 47951. No relevant comments were FEDERAL TRADE COMMISSION package can be obtained from the FTC received. Pursuant to the OMB [File No. 191 0061] Home Page (for November 15, 2019), on regulations, 5 CFR part 1320, that the World Wide Web, at https:// implement the PRA, 44 U.S.C. 3501 et Bristol-Myers Squibb Company and www.ftc.gov/news-events/commission- seq., the FTC is providing this second Celgene Corporation; Analysis of actions. opportunity for public comment while Agreement Containing Consent Orders You can file a comment online or on seeking OMB approval to renew the pre- To Aid Public Comment paper. For the Commission to consider existing clearance for those information your comment, we must receive it on or collection requirements. -

Complaint, “Chronic Pain” Means Non-Cancer Pain Lasting Three Months Or Longer
TABLE OF CONTENTS Page I. INTRODUCTION ...............................................................................................................1 II. JURISDICTION AND VENUE ..........................................................................................8 III. PARTIES .............................................................................................................................8 A. Plaintiff ....................................................................................................................8 B. Defendants ...............................................................................................................9 IV. FACTUAL ALLEGATIONS ............................................................................................14 A. Defendants Used Multiple Avenues To Disseminate Their False And Deceptive Statements About Opioids. ...................................................................14 1. Defendants spread and continue to spread their false and deceptive statements through direct marketing of their branded opioids. .......................................................................................................15 2. Defendants used a diverse group of seemingly independent third parties to spread false and deceptive statements about the risks and benefits of opioids.................................................................17 a. Key Opinion Leaders (“KOLs”) ....................................................19 (1) Russell Portenoy ................................................................20 -

Shire Acquisition of Viropharma - Strategic Move to Strengthen Shire’S Rare Disease Business - Augments Already Strong Growth Prospects
Shire acquisition of ViroPharma - Strategic move to strengthen Shire’s Rare Disease business - Augments already strong growth prospects Flemming Ornskov, MD Chief Executive Officer Graham Hetherington Chief Financial Officer Our purpose We enable people with life-altering conditions to lead better lives. CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS Statements included in this communication that are not historical facts are forward-looking statements. Forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: •Shire’s proposed acquisition of ViroPharma may not be consummated due to the occurrence of an event, change or other circumstances that gives rise to the termination of the merger agreement; •a governmental or regulatory approval required for the proposed acquisition of ViroPharma may not be obtained, or may be obtained subject to conditions that are not anticipated, or another condition to the closing of the proposed acquisition may not be satisfied; •ViroPharma may be unable to retain and hire key personnel and/or maintain its relationships with customers, suppliers and other business partners pending the consummation of the proposed acquisition by Shire, or ViroPharma’s business may be disrupted by the proposed acquisition, including increased costs and diversion of management time and resources; •difficulties in integrating ViroPharma into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; and risks and uncertainties detailed from time to time in Shire’s or ViroPharma’s filings with the U.S. -

Package Insert Has Been Approved by the U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION **Doses up to 1,000 U every 3 to 4 days may be considered based on These highlights do not include all the information needed to use individual patient response. ® CINRYZE safely and effectively. See full prescribing information for ---------------------- DOSAGE FORMS AND STRENGTHS -------------------- CINRYZE. Approximately 500 Units (lyophilized) in an 8 mL vial. (3) CINRYZE (C1 Esterase Inhibitor [Human]) ---------------------------- CONTRAINDICATIONS ------------------------------- For Intravenous Use, Freeze-Dried Powder for Reconstitution Patients who have manifested life-threatening immediate hypersensitivity Initial U.S. Approval: 2008. reactions, including anaphylaxis, to the product (4). --------------------------- RECENT MAJOR CHANGES -------------------------- ----------------------------- WARNINGS/PRECAUTIONS ------------------------ • Indications and Usage (1) 06/2018 • Hypersensitivity reactions may occur. Have epinephrine immediately • Dosage and Administration (2.1) 06/2018 available for treatment of acute severe hypersensitivity reaction (5.1) • --------------------------- INDICATIONS AND USAGE --------------------------- Serious arterial and venous thromboembolic (TE) events have been CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against reported at the recommended dose of C1 Esterase Inhibitor (Human) angioedema attacks in adults, adolescents and pediatric patients (6 years of products, including CINRYZE, following administration in patients with age and older) -

Representative Legal Matters Randall B
Representative Legal Matters Randall B. Sunberg H. Lundbeck A/S in the licensing to Denovo Biopharma of global rights to idalopirdine (an oral 5-HT6 antagonist) for Alzheimer's Disease, schizophrenia and other indications, subject to options for Lundbeck to re-acquire such rights with the parties sharing China rights. Takeda in its strategic collaboration with KSQ Therapeutics to identify optimal T cell and NK cell gene targets screened using KSQ’s CRISPRomics technology and to develop and commercialize novel cell and non-cell immuno-oncology therapies. NBE Therapeutics in its collaboration with Exelixis to discover and develop antibody-drug conjugates for oncology applications. Takeda in the sale to Neuraxpharm of global rights to the prescription brand Buccolam® (indicated for the emergency treatment of epileptic children with prolonged acute convulsive seizures). BeiGene in its collaboration with Assembly Biosciences to develop and commercialize in China, Hong Kong, Macau and Taiwan Assembly’s portfolio of three clinical-stage core inhibitor candidates for the treatment of patients with chronic hepatitis B virus (HBV) infection. GSK Consumer Healthcare in its collaboration with Mammoth Biosciences to develop a rapid, handheld CRISPR-based test to detect novel coronavirus infections. H. Lundbeck A/S in the acquisition of Alder BioPharmaceuticals, Inc, a company committed to migraine treatment and prevention, a transaction valued at USD 1.95 billion. Galapagos in its transformative USD 5.1 billion research and development collaboration -

The Path to $10 Billion in Product Sales by 2020
10 x 20: The Path to $10 Billion in Product Sales by 2020 Flemming Ornskov, MD, MPH CEO James Bowling Interim CFO The "SAFE HARBOR“ Statement under the Private Securities Litigation Reform Act of 1995 Statements included in this announcement that are not historical facts are forward-looking statements. Forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, Shire’s results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: . Shire’s products may not be a commercial success; . revenues from ADDERALL XR® are subject to generic erosion and revenues from INTUNIV® will become subject to generic competition starting in December 2014; . the failure to obtain and maintain reimbursement, or an adequate level of reimbursement, by third-party payors in a timely manner for Shire's products may impact future revenues, financial condition and results of operations; . Shire conducts its own manufacturing operations for certain of its Rare Diseases products and is reliant on third party contractors to manufacture other products and to provide goods and services. Some of Shire’s products or ingredients are only available from a single approved source for manufacture. Any disruption to the supply chain for any of Shire’s products may result in the Shire being unable to continue marketing or developing a product or may result in Shire being unable to do so on a commercially viable basis for some period of time. the development, approval and manufacturing of Shire’s products is subject to extensive oversight by various regulatory agencies. -

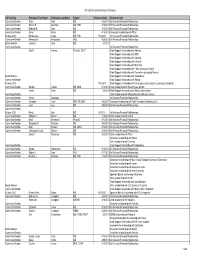
FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc.