The Path to $10 Billion in Product Sales by 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -
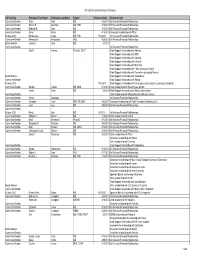
CB2013 Committee and Board Disclosureauditlist 9.10.13
2013-2014 Conflict of Interest Disclosure AST Activity Participant First Name Participant Last Name Degree DisclosureDate Disclsoure Item Committee Member Reza Abdi MD 4/22/2013 No Relevant Financial Relationships Committee Member Sameh R. Abul-Ezz MD, PhD 4/16/2013 No Relevant Financial Relationships Committee Member Deborah B. Adey MD 4/15/2013 No Relevant Financial Relationships Committee Member Enver Akalin MD 4/16/2013 Consultant relationship with Pfizer Fellows 2013 Maria-Luisa Alegre MD, PhD 7/12/2013 No Relevant Financial Relationships Committee Member Alessandrini Alessandro PhD 4/29/2013 No Relevant Financial Relationships Board Member James S. Allan MD 5/3/2013 Committee Member No Relevant Financial Relationships Rita R. Alloway PharmD, FCCP Grant Support relationship with Astellas Grant Support relationship with BMS Grant Support relationship with Novartis Grant Support relationship with Lifecycle Grant Support relationship with Millenium Grant Support relationship with Pfizer (previously Wyeth) Grant Support relationship with Genentech (previously Roche) Board Member Grant Support relationship with Viropharma Committee Member Grant Support relationship with Alexion Fellows 2013 7/18/2013 Grant Support relationship with Sanofi (previously Genzyme, previously Sangstat) Committee Member Sandra Amaral MD, MHS 6/18/2013 Other relationship with Bristol Myers-Squibb Hatem Amer MD 5/6/2013 Grant Support relationship with Abbott Laborartories. Committee Member Other relationship with Massacheussetts Medical Society. Committee Member William Applegate No Relevant Financial Relationships Committee Member Alexander Aussi BSN, RN, MBA 4/22/2013 Consultant relationship with Total Transplant Advantage, LLC Committee Member Jamil Azzi MD 4/30/2013 No Relevant Financial Relationships Committee Member Fellows 2013 Mark L. -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

Federal Register/Vol. 84, No. 232/Tuesday, December 3, 2019
Federal Register / Vol. 84, No. 232 / Tuesday, December 3, 2019 / Notices 66191 the Assistant Attorney General, patterns, devices, manufacturing filed with and accepted, subject to final developed the HSR Rules and the processes, or customer names. approval, by the Commission, has been corresponding Notification and Report placed on the public record for a period Form. Heather Hippsley, of thirty (30) days. The following On September 11, 2019, the Deputy General Counsel. Analysis to Aid Public Comment Commission sought comment on the [FR Doc. 2019–26075 Filed 12–2–19; 8:45 am] describes the terms of the consent reporting requirements associated with BILLING CODE 6750–01–P agreement and the allegations in the the HSR Rules and corresponding complaint. An electronic copy of the Notification and Report Form. 84 FR full text of the consent agreement 47951. No relevant comments were FEDERAL TRADE COMMISSION package can be obtained from the FTC received. Pursuant to the OMB [File No. 191 0061] Home Page (for November 15, 2019), on regulations, 5 CFR part 1320, that the World Wide Web, at https:// implement the PRA, 44 U.S.C. 3501 et Bristol-Myers Squibb Company and www.ftc.gov/news-events/commission- seq., the FTC is providing this second Celgene Corporation; Analysis of actions. opportunity for public comment while Agreement Containing Consent Orders You can file a comment online or on seeking OMB approval to renew the pre- To Aid Public Comment paper. For the Commission to consider existing clearance for those information your comment, we must receive it on or collection requirements. -

Shire Acquisition of Viropharma - Strategic Move to Strengthen Shire’S Rare Disease Business - Augments Already Strong Growth Prospects
Shire acquisition of ViroPharma - Strategic move to strengthen Shire’s Rare Disease business - Augments already strong growth prospects Flemming Ornskov, MD Chief Executive Officer Graham Hetherington Chief Financial Officer Our purpose We enable people with life-altering conditions to lead better lives. CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS Statements included in this communication that are not historical facts are forward-looking statements. Forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: •Shire’s proposed acquisition of ViroPharma may not be consummated due to the occurrence of an event, change or other circumstances that gives rise to the termination of the merger agreement; •a governmental or regulatory approval required for the proposed acquisition of ViroPharma may not be obtained, or may be obtained subject to conditions that are not anticipated, or another condition to the closing of the proposed acquisition may not be satisfied; •ViroPharma may be unable to retain and hire key personnel and/or maintain its relationships with customers, suppliers and other business partners pending the consummation of the proposed acquisition by Shire, or ViroPharma’s business may be disrupted by the proposed acquisition, including increased costs and diversion of management time and resources; •difficulties in integrating ViroPharma into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; and risks and uncertainties detailed from time to time in Shire’s or ViroPharma’s filings with the U.S. -

Package Insert Has Been Approved by the U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION **Doses up to 1,000 U every 3 to 4 days may be considered based on These highlights do not include all the information needed to use individual patient response. ® CINRYZE safely and effectively. See full prescribing information for ---------------------- DOSAGE FORMS AND STRENGTHS -------------------- CINRYZE. Approximately 500 Units (lyophilized) in an 8 mL vial. (3) CINRYZE (C1 Esterase Inhibitor [Human]) ---------------------------- CONTRAINDICATIONS ------------------------------- For Intravenous Use, Freeze-Dried Powder for Reconstitution Patients who have manifested life-threatening immediate hypersensitivity Initial U.S. Approval: 2008. reactions, including anaphylaxis, to the product (4). --------------------------- RECENT MAJOR CHANGES -------------------------- ----------------------------- WARNINGS/PRECAUTIONS ------------------------ • Indications and Usage (1) 06/2018 • Hypersensitivity reactions may occur. Have epinephrine immediately • Dosage and Administration (2.1) 06/2018 available for treatment of acute severe hypersensitivity reaction (5.1) • --------------------------- INDICATIONS AND USAGE --------------------------- Serious arterial and venous thromboembolic (TE) events have been CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against reported at the recommended dose of C1 Esterase Inhibitor (Human) angioedema attacks in adults, adolescents and pediatric patients (6 years of products, including CINRYZE, following administration in patients with age and older) -

Representative Legal Matters Randall B
Representative Legal Matters Randall B. Sunberg H. Lundbeck A/S in the licensing to Denovo Biopharma of global rights to idalopirdine (an oral 5-HT6 antagonist) for Alzheimer's Disease, schizophrenia and other indications, subject to options for Lundbeck to re-acquire such rights with the parties sharing China rights. Takeda in its strategic collaboration with KSQ Therapeutics to identify optimal T cell and NK cell gene targets screened using KSQ’s CRISPRomics technology and to develop and commercialize novel cell and non-cell immuno-oncology therapies. NBE Therapeutics in its collaboration with Exelixis to discover and develop antibody-drug conjugates for oncology applications. Takeda in the sale to Neuraxpharm of global rights to the prescription brand Buccolam® (indicated for the emergency treatment of epileptic children with prolonged acute convulsive seizures). BeiGene in its collaboration with Assembly Biosciences to develop and commercialize in China, Hong Kong, Macau and Taiwan Assembly’s portfolio of three clinical-stage core inhibitor candidates for the treatment of patients with chronic hepatitis B virus (HBV) infection. GSK Consumer Healthcare in its collaboration with Mammoth Biosciences to develop a rapid, handheld CRISPR-based test to detect novel coronavirus infections. H. Lundbeck A/S in the acquisition of Alder BioPharmaceuticals, Inc, a company committed to migraine treatment and prevention, a transaction valued at USD 1.95 billion. Galapagos in its transformative USD 5.1 billion research and development collaboration -

Non-Merger Civil Enforcement: an Overview of Recent DOJ and FTC Federal Court Litigation
Antitrust , Vol. 32, No. 1, Fall 201 7. © 2017 by the American Bar Association. Reproduced with permission. All rights reserved. This information or any portion thereof may not be copied or disseminated in any form or by any means or stored in an electronic database or retrieval system without the express written consent of the American Bar Association. Non-Merger Civil Enforcement: An Overview of Recent DOJ and FTC Federal Court Litigation BY SONIA KUESTER PFAFFENROTH ECENT YEARS HAVE SEEN THE As a result, there are now a significant number of career attor - Department of Justice and the Federal Trade neys and economists with recent federal trial court experience, Commission appearing with regularity in fed - which they will bring to future cases at the investigative phase eral district court, with the agencies demon - with an eye towards potential litigation. strating a willingness to litigate in both the Rmerger and non-merger context and with a number of high- DOJ Litigation profile trials now in the rearview mirror. Because the DOJ has no administrative adjudicative process, While the majority of civil conduct enforcement actions its civil enforcement cases, whether they are settlements or continue to be filed concurrently with settlements—which contested litigation, are filed directly in federal district court. provide significant insight into the government’s theories— In recent years, the DOJ has litigated a number of cases alleg - both agencies have seen an uptick in the number of contest - ing Section 1 violations and one case alleging a Section 2 vio - ed cases filed in federal district court since the beginning of lation. -

Surprises in the Industry's Most Profitable Companies
June 20, 2008 Surprises in the industry's most profitable companies Evaluate Vantage An analysis of the industry’s most profitable companies last year throws up some surprising names within the top ten – ViroPharma tops the league table with a net income margin of 47%, generating all its profits from sales of anti-bacterial agent Vancocin. Interestingly, two Indian generic firms feature, Sun Pharmaceutical Industries and Glenmark Pharmaceuticals, perhaps dispelling the notion that margins from selling generics are always low. Of the big pharma players, only those US groups with mature product portfolios, namely Pfizer, Merck & Co and Amgen, make it into the list. However, looking at forecast margins for the current year, the table below also reveals that Merck & Co drops out of the top ten, impacted by significantly lower sales from its cholesterol franchise. Alcon replaces Merck, suggesting one good reason why Novartis recently decided to fork out $11bn to acquire a 25% stake in the eye-care company. This analysis from EvaluatePharma is based on dividing normalised net income (excluding exceptional items) by the total revenues, to generate a net margin percentage. It only includes companies that have reported two consecutive years of profit, with incomes in excess of $50m in both 2007 and 2008. 2007 - top 10 companies Net Margin Net Income - Normalised ($m) Total Revenues ($m) 2007 2006 2007 2007 ViroPharma 47% 66 95 204 Sun Pharmaceutical Industries 44% 159 371 837 Gilead Sciences 38% 1,204 1,615 4,230 Biovail 35% 428 293 843 OSI Pharmaceuticals -

Biopartnering Executive Summary
BIOWORLD® BIOPARTNERING REPORT 2009: STRATEGIES AND PARADIGMS OF THE DEAL EXECUTIVE SUMMARY By Michael Harris, BioWorld Executive Editor and Amanda Lyle, BioWorld Managing Editor LIST OF CONTENTS Introduction and Analysis Trends Depressing First Half of 2008 in Worldwide Public Markets Drug/Device Combos Could Salvage Dropped Compounds A Match Made in Heaven; R&D Productivity Via Partnering Drug and Medical Device Deals: A Different Type of Partnership As Big Pharma Model Falters, Biotech Rides to the Rescue Deals to M&As: The Culture of False-Flagging, Shape-Shifting Notable Deals Autoimmune and Inflammation Deals Immunomedics Inks Potential $620M Deal with Nycomed ARYx Seeking New Partner for GI Program after P&G Bails Out UCB Licenses Keppra, Cimzia Rights in Japan Alizyme, Norgine Team Up in $67M Marketing Deal Bionomics Signs Merck Serono in MS, Autoimmune Partnership TransPharma Gets $35M in Osteo Deal with Lilly QLT Sells Acne Gel Product Aczone for $150M to Allergan Start-up TFT Adds NF-Kappa B Decoy Program from Anesiva Early Stage Antibody Nets EUSA $44M from GSK Scil, Pfizer Enter $250M Deal for Preclinical Osteo Drug Catalyst Partners with Centocor for Engineered Protease Drugs Galapagos Inks $395M Deal with Lilly for Osteo Drugs Alba Inks Ex-U.S./Japan Deal with Shire for Celiac Drug Excaliard Snags $15.5M and Isis Fibrosis Drugs Galapagos and Janssen in RA Partnership Axcan, Cellerix Deal for Adult Stem Cell Therapy Radius Grants Novartis Option to BA058 in Potential $500M Deal Microbia, Forest Collaborate: Up to $330M for -

Patient Services, Inc. 2014 Annual Report Table of Contents
Patient Services, Inc. 2014 Annual Report Table of ConTenTs 2014 Board of Directors and Sponsors .......................................................................................... 3 Pharmaceutical, Provider Industries, Corporate, Government and Individual Sponsors ............ 3-5 Ways to Support PSI ..................................................................................................................... 6 2014 Executive Message ................................................................................................................ 7 Marketing and Development ........................................................................................................ 8 Government Relations .................................................................................................................. 9 Operations and Program Reimbursement ....................................................................................10 PSI Traditional Programs ....................................................................................................... 11-13 IT Department ............................................................................................................................14 ACCESS® Program ......................................................................................................................15 Message from our Board Treasurer ..............................................................................................16 Statement of Activities .................................................................................................................17 -

1 United States District Court for the District Of
Case 1:12-cv-00584-ESH Document 33 Filed 04/23/12 Page 1 of 42 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA ViroPharma, Inc., Plaintiff, v. Margaret A. Hamburg, M.D., in her official capacity as Commissioner, Food and Drug Administration, et al., Civil Action No. 12-0584 (ESH) Defendants, and Akorn, Inc., et al., Defendants-intervenors. MEMORANDUM OPINION ViroPharma, Inc., manufactures the antibiotic Vancocin®. On April 13, 2012, ViroPharma sued Margaret Hamburg, in her official capacity as the Commissioner of the Food and Drug Administration; Kathleen Sebelius, in her official capacity as the Secretary of the Department of Health and Human Services; and the agencies themselves (collectively, the “FDA”) to challenge the FDA’s approval, on April 9, 2012, of three Abbreviated New Drug Applications (“ANDAs”) permitting the marketing of generic versions of Vancocin (vancomycin hydrochloride capsules or “vancomycin”). (See Complaint, April 13, 2012 [Dkt. No. 1] (“Compl.”).) ViroPharma alleges that the FDA approved the three ANDAs (1) in violation of ViroPharma’s statutory right under the Federal Food, Drug, and Cosmetic Act (“FFDCA”), 21 U.S.C. §§ 301 et seq., to a three-year period of exclusivity for Vancocin, extending through 1 Case 1:12-cv-00584-ESH Document 33 Filed 04/23/12 Page 2 of 42 December 15, 2014; and (2) based solely on in vitro (laboratory) bioequivalence testing in violation of the FDA’s own regulations requiring in vivo (human) bioequivalence testing. (Id. ¶ 2.) The Court will refer to these as ViroPharma’s “statutory exclusivity claim” (see id. ¶¶ 75–78 (Count II)) and its “bioequivalence claim.” (See id.