The Physics of Stars Class 3 Notes: Hydrostatic Balance and the Virial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrostatic Shapes
7 Hydrostatic shapes It is primarily the interplay between gravity and contact forces that shapes the macroscopic world around us. The seas, the air, planets and stars all owe their shape to gravity, and even our own bodies bear witness to the strength of gravity at the surface of our massive planet. What physics principles determine the shape of the surface of the sea? The sea is obviously horizontal at short distances, but bends below the horizon at larger distances following the planet’s curvature. The Earth as a whole is spherical and so is the sea, but that is only the first approximation. The Moon’s gravity tugs at the water in the seas and raises tides, and even the massive Earth itself is flattened by the centrifugal forces of its own rotation. Disregarding surface tension, the simple answer is that in hydrostatic equi- librium with gravity, an interface between two fluids of different densities, for example the sea and the atmosphere, must coincide with a surface of constant ¡A potential, an equipotential surface. Otherwise, if an interface crosses an equipo- ¡ A ¡ A tential surface, there will arise a tangential component of gravity which can only ¡ ©¼AAA be balanced by shear contact forces that a fluid at rest is unable to supply. An ¡ AAA ¡ g AUA iceberg rising out of the sea does not obey this principle because it is solid, not ¡ ? A fluid. But if you try to build a little local “waterberg”, it quickly subsides back into the sea again, conforming to an equipotential surface. Hydrostatic balance in a gravitational field also implies that surfaces of con- A triangular “waterberg” in the sea. -
10. Collisions • Use Conservation of Momentum and Energy and The
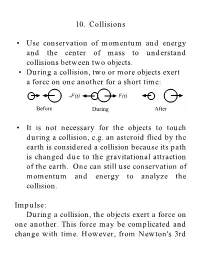
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Dark Matter 1 Introduction 2 Evidence of Dark Matter
Proceedings Astronomy from 4 perspectives 1. Cosmology Dark matter Marlene G¨otz (Jena) Dark matter is a hypothetical form of matter. It has to be postulated to describe phenomenons, which could not be explained by known forms of matter. It has to be assumed that the largest part of dark matter is made out of heavy, slow moving, electric and color uncharged, weakly interacting particles. Such a particle does not exist within the standard model of particle physics. Dark matter makes up 25 % of the energy density of the universe. The true nature of dark matter is still unknown. 1 Introduction Due to the cosmic background radiation the matter budget of the universe can be divided into a pie chart. Only 5% of the energy density of the universe is made of baryonic matter, which means stars and planets. Visible matter makes up only 0,5 %. The influence of dark matter of 26,8 % is much larger. The largest part of about 70% is made of dark energy. Figure 1: The universe in a pie chart [1] But this knowledge was obtained not too long ago. At the beginning of the 20th century the distribution of luminous matter in the universe was assumed to correspond precisely to the universal mass distribution. In the 1920s the Caltech professor Fritz Zwicky observed something different by looking at the neighbouring Coma Cluster of galaxies. When he measured what the motions were within the cluster, he got an estimate for how much mass there was. Then he compared it to how much mass he could actually see by looking at the galaxies. -

AST242 LECTURE NOTES PART 3 Contents 1. Viscous Flows 2 1.1. the Velocity Gradient Tensor 2 1.2. Viscous Stress Tensor 3 1.3. Na
AST242 LECTURE NOTES PART 3 Contents 1. Viscous flows 2 1.1. The velocity gradient tensor 2 1.2. Viscous stress tensor 3 1.3. Navier Stokes equation { diffusion 5 1.4. Viscosity to order of magnitude 6 1.5. Example using the viscous stress tensor: The force on a moving plate 6 1.6. Example using the Navier Stokes equation { Poiseuille flow or Flow in a capillary 7 1.7. Viscous Energy Dissipation 8 2. The Accretion disk 10 2.1. Accretion to order of magnitude 14 2.2. Hydrostatic equilibrium for an accretion disk 15 2.3. Shakura and Sunyaev's α-disk 17 2.4. Reynolds Number 17 2.5. Circumstellar disk heated by stellar radiation 18 2.6. Viscous Energy Dissipation 20 2.7. Accretion Luminosity 21 3. Vorticity and Rotation 24 3.1. Helmholtz Equation 25 3.2. Rate of Change of a vector element that is moving with the fluid 26 3.3. Kelvin Circulation Theorem 28 3.4. Vortex lines and vortex tubes 30 3.5. Vortex stretching and angular momentum 32 3.6. Bernoulli's constant in a wake 33 3.7. Diffusion of vorticity 34 3.8. Potential Flow and d'Alembert's paradox 34 3.9. Burger's vortex 36 4. Rotating Flows 38 4.1. Coriolis Force 38 4.2. Rossby and Ekman numbers 39 4.3. Geostrophic flows and the Taylor Proudman theorem 39 4.4. Two dimensional flows on the surface of a planet 41 1 2 AST242 LECTURE NOTES PART 3 4.5. Thermal winds? 42 5. -

Energy Literacy Essential Principles and Fundamental Concepts for Energy Education
Energy Literacy Essential Principles and Fundamental Concepts for Energy Education A Framework for Energy Education for Learners of All Ages About This Guide Energy Literacy: Essential Principles and Intended use of this document as a guide includes, Fundamental Concepts for Energy Education but is not limited to, formal and informal energy presents energy concepts that, if understood and education, standards development, curriculum applied, will help individuals and communities design, assessment development, make informed energy decisions. and educator trainings. Energy is an inherently interdisciplinary topic. Development of this guide began at a workshop Concepts fundamental to understanding energy sponsored by the Department of Energy (DOE) arise in nearly all, if not all, academic disciplines. and the American Association for the Advancement This guide is intended to be used across of Science (AAAS) in the fall of 2010. Multiple disciplines. Both an integrated and systems-based federal agencies, non-governmental organizations, approach to understanding energy are strongly and numerous individuals contributed to the encouraged. development through an extensive review and comment process. Discussion and information Energy Literacy: Essential Principles and gathered at AAAS, WestEd, and DOE-sponsored Fundamental Concepts for Energy Education Energy Literacy workshops in the spring of 2011 identifies seven Essential Principles and a set of contributed substantially to the refinement of Fundamental Concepts to support each principle. the guide. This guide does not seek to identify all areas of energy understanding, but rather to focus on those To download this guide and related documents, that are essential for all citizens. The Fundamental visit www.globalchange.gov. Concepts have been drawn, in part, from existing education standards and benchmarks. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis
Energy & Society Energy Units and Fundamentals Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis 1 Energy & Society Energy Units and Fundamentals Table of Contents 1. Key Concepts: Force, Work, Energy & Power 3 2. Orders of Magnitude & Scientific Notation 6 2.1. Orders of Magnitude 6 2.2. Scientific Notation 7 2.3. Rules for Calculations 7 2.3.1. Multiplication 8 2.3.2. Division 8 2.3.3. Exponentiation 8 2.3.4. Square Root 8 2.3.5. Addition & Subtraction 9 3. Linear versus Exponential Growth 10 3.1. Linear Growth 10 3.2. Exponential Growth 11 4. Uncertainty & Significant Figures 14 4.1. Uncertainty 14 4.2. Significant Figures 15 4.3. Exact Numbers 15 4.4. Identifying Significant Figures 16 4.5. Rules for Calculations 17 4.5.1. Addition & Subtraction 17 4.5.2. Multiplication, Division & Exponentiation 18 5. Unit Analysis 19 5.1. Commonly Used Energy & Non-energy Units 20 5.2. Form & Function 21 6. Sample Problems 22 6.1. Scientific Notation 22 6.2. Linear & Exponential Growth 22 6.3. Significant Figures 23 6.4. Unit Conversions 23 7. Answers to Sample Problems 24 7.1. Scientific Notation 24 7.2. Linear & Exponential Growth 24 7.3. Significant Figures 24 7.4. Unit Conversions 26 8. References 27 2 Energy & Society Energy Units and Fundamentals 1. KEY CONCEPTS: FORCE, WORK, ENERGY & POWER Among the most important fundamentals to be mastered when studying energy pertain to the differences and inter-relationships among four concepts: force, work, energy, and power. Each of these terms has a technical meaning in addition to popular or colloquial meanings. -

Astronomy 201 Review 2 Answers What Is Hydrostatic Equilibrium? How Does Hydrostatic Equilibrium Maintain the Su
Astronomy 201 Review 2 Answers What is hydrostatic equilibrium? How does hydrostatic equilibrium maintain the Sun©s stable size? Hydrostatic equilibrium, also known as gravitational equilibrium, describes a balance between gravity and pressure. Gravity works to contract while pressure works to expand. Hydrostatic equilibrium is the state where the force of gravity pulling inward is balanced by pressure pushing outward. In the core of the Sun, hydrogen is being fused into helium via nuclear fusion. This creates a large amount of energy flowing from the core which effectively creates an outward-pushing pressure. Gravity, on the other hand, is working to contract the Sun towards its center. The outward pressure of hot gas is balanced by the inward force of gravity, and not just in the core, but at every point within the Sun. What is the Sun composed of? Explain how the Sun formed from a cloud of gas. Why wasn©t the contracting cloud of gas in hydrostatic equilibrium until fusion began? The Sun is primarily composed of hydrogen (70%) and helium (28%) with the remaining mass in the form of heavier elements (2%). The Sun was formed from a collapsing cloud of interstellar gas. Gravity contracted the cloud of gas and in doing so the interior temperature of the cloud increased because the contraction converted gravitational potential energy into thermal energy (contraction leads to heating). The cloud of gas was not in hydrostatic equilibrium because although the contraction produced heat, it did not produce enough heat (pressure) to counter the gravitational collapse and the cloud continued to collapse. -

STARS in HYDROSTATIC EQUILIBRIUM Gravitational Energy
STARS IN HYDROSTATIC EQUILIBRIUM Gravitational energy and hydrostatic equilibrium We shall consider stars in a hydrostatic equilibrium, but not necessarily in a thermal equilibrium. Let us define some terms: U = kinetic, or in general internal energy density [ erg cm −3], (eql.1a) U u ≡ erg g −1 , (eql.1b) ρ R M 2 Eth ≡ U4πr dr = u dMr = thermal energy of a star, [erg], (eql.1c) Z Z 0 0 M GM dM Ω= − r r = gravitational energy of a star, [erg], (eql.1d) Z r 0 Etot = Eth +Ω = total energy of a star , [erg] . (eql.1e) We shall use the equation of hydrostatic equilibrium dP GM = − r ρ, (eql.2) dr r and the relation between the mass and radius dM r =4πr2ρ, (eql.3) dr to find a relations between thermal and gravitational energy of a star. As we shall be changing variables many times we shall adopt a convention of using ”c” as a symbol of a stellar center and the lower limit of an integral, and ”s” as a symbol of a stellar surface and the upper limit of an integral. We shall be transforming an integral formula (eql.1d) so, as to relate it to (eql.1c) : s s s GM dM GM GM ρ Ω= − r r = − r 4πr2ρdr = − r 4πr3dr = (eql.4) Z r Z r Z r2 c c c s s s dP s 4πr3dr = 4πr3dP =4πr3P − 12πr2P dr = Z dr Z c Z c c c s −3 P 4πr2dr =Ω. Z c Our final result: gravitational energy of a star in a hydrostatic equilibrium is equal to three times the integral of pressure within the star over its entire volume. -

1. A) the Sun Is in Hydrostatic Equilibrium. What Does
AST 301 Test #3 Friday Nov. 12 Name:___________________________________ 1. a) The Sun is in hydrostatic equilibrium. What does this mean? What is the definition of hydrostatic equilibrium as we apply it to the Sun? Pressure inside the star pushing it apart balances gravity pulling it together. So it doesn’t change its size. 1. a) The Sun is in thermal equilibrium. What does this mean? What is the definition of thermal equilibrium as we apply it to the Sun? Energy generation by nuclear fusion inside the star balances energy radiated from the surface of the star. So it doesn’t change its temperature. b) Give an example of an equilibrium (not necessarily an astronomical example) different from hydrostatic and thermal equilibrium. Water flowing into a bucket balances water flowing out through a hole. When supply and demand are equal the price is stable. The floor pushes me up to balance gravity pulling me down. 2. If I want to use the Doppler technique to search for a planet orbiting around a star, what measurements or observations do I have to make? I would measure the Doppler shift in the spectrum of the star caused by the pull of the planet’s gravity on the star. 2. If I want to use the transit (or eclipse) technique to search for a planet orbiting around a star, what measurements or observations do I have to make? I would observe the dimming of the star’s light when the planet passes in front of the star. If I find evidence of a planet this way, describe some information my measurements give me about the planet. -

Heat and Energy Conservation
1 Lecture notes in Fluid Dynamics (1.63J/2.01J) by Chiang C. Mei, MIT, Spring, 2007 CHAPTER 4. THERMAL EFFECTS IN FLUIDS 4-1-2energy.tex 4.1 Heat and energy conservation Recall the basic equations for a compressible fluid. Mass conservation requires that : ρt + ∇ · ρ~q = 0 (4.1.1) Momentum conservation requires that : = ρ (~qt + ~q∇ · ~q)= −∇p + ∇· τ +ρf~ (4.1.2) = where the viscous stress tensor τ has the components = ∂qi ∂qi ∂qk τ = τij = µ + + λ δij ij ∂xj ∂xi ! ∂xk There are 5 unknowns ρ, p, qi but only 4 equations. One more equation is needed. 4.1.1 Conservation of total energy Consider both mechanical ad thermal energy. Let e be the internal (thermal) energy per unit mass due to microscopic motion, and q2/2 be the kinetic energy per unit mass due to macroscopic motion. Conservation of energy requires D q2 ρ e + dV rate of incr. of energy in V (t) Dt ZZZV 2 ! = − Q~ · ~ndS rate of heat flux into V ZZS + ρf~ · ~qdV rate of work by body force ZZZV + Σ~ · ~qdS rate of work by surface force ZZX Use the kinematic transport theorm, the left hand side becomes D q2 ρ e + dV ZZZV Dt 2 ! 2 Using Gauss theorem the heat flux term becomes ∂Qi − QinidS = − dV ZZS ZZZV ∂xi The work done by surface stress becomes Σjqj dS = (σjini)qj dS ZZS ZZS ∂(σijqj) = (σijqj)ni dS = dV ZZS ZZZV ∂xi Now all terms are expressed as volume integrals over an arbitrary material volume, the following must be true at every point in space, 2 D q ∂Qi ∂(σijqi) ρ e + = − + ρfiqi + (4.1.3) Dt 2 ! ∂xi ∂xj As an alternative form, we differentiate the kinetic energy and get De -

Lecture 5: Magnetic Mirroring
!"#$%&'()%"*#%*+,-./-*+01.2(.*3+456789* !"#$%&"'()'*+,-".#'*/&&0&/-,' Dr. Peter T. Gallagher Astrophysics Research Group Trinity College Dublin :&2-;-)(*!"<-$2-"(=*%>*/-?"=)(*/%/="#* o Gyrating particle constitutes an electric current loop with a dipole moment: 1/2mv2 µ = " B o The dipole moment is conserved, i.e., is invariant. Called the first adiabatic invariant. ! o µ = constant even if B varies spatially or temporally. If B varies, then vperp varies to keep µ = constant => v|| also changes. o Gives rise to magnetic mirroring. Seen in planetary magnetospheres, magnetic bottles, coronal loops, etc. Bz o Right is geometry of mirror Br from Chen, Page 30. @-?"=)(*/2$$%$2"?* o Consider B-field pointed primarily in z-direction and whose magnitude varies in z- direction. If field is axisymmetric, B! = 0 and d/d! = 0. o This has cylindrical symmetry, so write B = Brrˆ + Bzzˆ o How does this configuration give rise to a force that can trap a charged particle? ! o Can obtain Br from " #B = 0 . In cylindrical polar coordinates: 1 " "Bz (rBr )+ = 0 r "r "z ! " "Bz => (rBr ) = #r "r "z o If " B z / " z is given at r = 0 and does not vary much with r, then r $Bz 1 2&$ Bz ) ! rBr = " #0 r dr % " r ( + $z 2 ' $z *r =0 ! 1 &$ Bz ) Br = " r( + (5.1) 2 ' $z *r =0 ! @-?"=)(*/2$$%$2"?* o Now have Br in terms of BZ, which we can use to find Lorentz force on particle. o The components of Lorentz force are: Fr = q(v" Bz # vzB" ) (1) F" = q(#vrBz + vzBr ) (2) (3) Fz = q(vrB" # v" Br ) (4) o As B! = 0, two terms vanish and terms (1) and (2) give rise to Larmor gyration.