On the Analysis of Energy Efficient Aircraft Engines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jet Propulsion Engines
JET PROPULSION ENGINES 5.1 Introduction Jet propulsion, similar to all means of propulsion, is based on Newton’s Second and Third laws of motion. The jet propulsion engine is used for the propulsion of aircraft, m issile and submarine (for vehicles operating entirely in a fluid) by the reaction of jet of gases which are discharged rearw ard (behind) with a high velocity. A s applied to vehicles operating entirely in a fluid, a momentum is imparted to a mass of fluid in such a manner that the reaction of the imparted momentum furnishes a propulsive force. The magnitude of this propulsive force is termed as thrust. For efficient production of large power, fuel is burnt in an atmosphere of compressed air (combustion chamber), the products of combustion expanding first in a gas turbine which drives the air compressor and then in a nozzle from which the thrust is derived. Paraffin is usually adopted as the fuel because of its ease of atomisation and its low freezing point. Jet propulsion was utilized in the flying Bomb, the initial compression of the air being due to a divergent inlet duct in which a sm all increase in pressure energy w as obtained at the expense of kinetic energy of the air. Because of this very limited compression, the thermal efficiency of the unit was low, although huge power was obtained. In the normal type of jet propulsion unit a considerable improvement in efficiency is obtained by fitting a turbo-com pressor which w ill give a com pression ratio of at least 4:1. -

04 Propulsion
Aircraft Design Lecture 2: Aircraft Propulsion G. Dimitriadis and O. Léonard APRI0004-1, Aerospace Design Project, Lecture 4 1 Introduction • A large variety of propulsion methods have been used from the very start of the aerospace era: – No propulsion (balloons, gliders) – Muscle (mostly failed) – Steam power (mostly failed) – Piston engines and propellers – Rocket engines – Jet engines – Pulse jet engines – Ramjet – Scramjet APRI0004-1, Aerospace Design Project, Lecture 4 2 Gliding flight • People have been gliding from the- mid 18th century. The Albatross II by Jean Marie Le Bris- 1849 Otto Lillienthal , 1895 APRI0004-1, Aerospace Design Project, Lecture 4 3 Human-powered flight • Early attempts were less than successful but better results were obtained from the 1960s onwards. Gerhardt Cycleplane (1923) MIT Daedalus (1988) APRI0004-1, Aerospace Design Project, Lecture 4 4 Steam powered aircraft • Mostly dirigibles, unpiloted flying models and early aircraft Clément Ader Avion III (two 30hp steam engines, 1897) Giffard dirigible (1852) APRI0004-1, Aerospace Design Project, Lecture 4 5 Engine requirements • A good aircraft engine is characterized by: – Enough power to fulfill the mission • Take-off, climb, cruise etc. – Low weight • High weight increases the necessary lift and therefore the drag. – High efficiency • Low efficiency increases the amount fuel required and therefore the weight and therefore the drag. – High reliability – Ease of maintenance APRI0004-1, Aerospace Design Project, Lecture 4 6 Piston engines • Wright Flyer: One engine driving two counter- rotating propellers (one port one starboard) via chains. – Four in-line cylinders – Power: 12hp – Weight: 77 kg APRI0004-1, Aerospace Design Project, Lecture 4 7 Piston engine development • During the first half of the 20th century there was considerable development of piston engines. -

INTEGRATING SOLAR CELLS INTO COMPOSITE MATERIAL an Opportunity for Electric Planes and Uavs
“The future of aviation is electric” Dr Susan Ying, President of the International Council of Aeronautical Science and former Director of Boeing Research and Technology. WHITEPAPER INTEGRATING SOLAR CELLS INTO COMPOSITE MATERIAL An Opportunity for Electric Planes and UAVs CONTENTS EXECUTIVE SUMMARY Executive summary ..........................1 It is well established that adding solar cells to UAVs and manned electric aircraft is beneficial – the sun is an inexpensive, renewable, and plentiful What is holding back source of energy. So if the advantage of solar is clear, why don’t we see the solar aircraft industry?..............1 solar aircraft in the market? Electric aircraft revolution ..............2 Industry opinion is that a solution is required to enable drones to fly as long as needed. That solution has not been forthcoming. The approach The limiting factor has to date has been to glue solar cells on top of aircraft wings, but this is been battery technology .................3 inefficient, adds weight, and is not particularly aerodynamic. Solar cells are fragile and so far there has been no way to integrate solar cells into Challenging the status quo .............3 wing materials that was robust, inexpensive and light. Solar cell composites - Praxis Aeronautics deduced – from its own experience in composite a solution .............................................4 and aeronautical engineering – that what the industry needed was an aerodynamic solution that would enable solar cells to be shaped and From invention to reality ..................5 integrated into the product. The solution would need to make the cells flexible and able to efficiently absorb light. Praxis Aeronautics has Conclusion ..........................................6 developed a solution that meets these criteria. -

The Performance Economy: 2Nd Edition
9780230_584662_01_prexxiv.pdf 1/12/10 2:41 PM Page i The Performance Economy 9780230_584662_01_prexxiv.pdf 1/12/10 2:41 PM Page ii Also by Walter Stahel COMMON UTILIZATION INSTEAD OF SINGULAR CONSUMPTION – A NEW RELATIONSHIP WITH GOODS Stahel, Walter R. and Gomringer, Eugen (editors) ECONOMIC STRATEGY OF DURABILITY Börlin, Max and Stahel, Walter R. (authors) HANDBUCH VON BEISPIELEN EINER HÖHEREN RESSOURCEN-EFFIZIENZ Stahel, Walter R. (author) JOBS FOR TOMORROW, THE POTENTIAL FOR SUBSTITUTING MANPOWER FOR ENERGY Stahel, Walter R. and Reday, Geneviève (authors) LANGLEBIGKEIT UND MATERIALRECYCLING Stahel, Walter R. (author) RESSOURCENPRODUKTIVITÄT DURCH NUTZUNGSINTENSIVIERUNG UND LEBENSDAUERVERLÄNGERUNG Stahel, Walter R. (author) THE LIMITS TO CERTAINTY, FACING RISKS IN THE NEW SERVICE ECONOMY Giarini, Orio and Stahel, Walter R. (authors) Translated into French, Italian, Rumanian, Japanese and German 9780230_584662_01_prexxiv.pdf 1/12/10 2:41 PM Page iii The Performance Economy 2nd Edition Walter R. Stahel 9780230_584662_01_prexxiv.pdf 1/12/10 2:41 PM Page iv © Walter R. Stahel 2006, 2010 All rights reserved. No reproduction, copy or transmission of this publication may be made without written permission. No portion of this publication may be reproduced, copied or transmitted save with written permission or in accordance with the provisions of the Copyright, Designs and Patents Act 1988, or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, Saffron House, 6–10 Kirby Street, London EC1N 8TS. Any person who does any unauthorized act in relation to this publication may be liable to criminal prosecution and civil claims for damages. The author has asserted his right to be identified as the author of this work in accordance with the Copyright, Designs and Patents Act 1988. -

An Online Performance Calculator for Ramjet, Turbojet, Turbofan, and Turboprop Engines
An Online Performance Calculator for Ramjet, Turbojet, Turbofan, and Turboprop Engines Evan P. Warner∗ Virginia Tech, Blacksburg, VA, 24061, USA This paper documents the theory, equations, and assumptions behind an HTML-based online performance calculator for ramjet, turbojet, turbofan, and turboprop engines. Based on inputs of design parameters, flight con- ditions, and engine parameters, the specific thrust (()), thrust specific fuel consumption ()(), propulsive efficiency ([ ?), thermal efficiency ([Cℎ), and overall efficiency ([0) will be output for the engine of interest. Several sample cases are analyzed to verify the functionality of the calculator. These sample cases are derived from engines associated with Virginia Tech, which include: Pratt & Whitney’s PT6A-20 and JT15D-1, Honeywell’s TFE731-2B, and the Rolls Royce Trent 1000. With the help of this calculator, students will now be able to verify their air-breathing jet engine performance value calculations. This calculator is available here. I. Nomenclature "0 = Ambient Mach Number 2 ?1 = Specific Heat of the Burner W0 = Ambient Specific Heat Ratio 2 ?C = Specific Heat of the Turbine W3 = Diffuser Specific Heat Ratio 2 ? 5 = Specific Heat of the Fan W2 = Compressor Specific Heat Ratio )0 = Ambient Static Temperature W1 = Burner Specific Heat Ratio ?0 = Ambient Static Pressure WC = Turbine Specific Heat Ratio ?4 = Exit Static Pressure W= = Nozzle Specific Heat Ratio '08A = Gas Constant for Air W 5 = Fan Specific Heat Ratio '?A>3 = Gas Constant for the Products of Fuel/Air Mixture W= 5 = Fan Nozzle -

Hybrid Propulsion System of a Long Endurance Electric UAV
Hybrid Propulsion System of a Long Endurance Electric UAV Tiago Miguel Moreira Ferreira Thesis to obtain the Master of Science Degree in Mechanical Engineering Supervisor(s): Prof. André Calado Marta Prof. Virginia Isabel Monteiro Nabais Infante Examination Committee Chairperson: Prof. Luís Manuel Varejão Oliveira Faria Supervisor: Prof. André Calado Marta Member of the Committee: Prof. António Manuel Relógio Ribeiro November 2014 ii Acknowledgments I would like to thank my parents, Gracinda Ferreira and Mario´ Ferreira, for supporting me along my academic path. I would also like to thank Daniela Carvalho for always being there to help me along the way. Thanks to the professors, Dr. Andre´ Marta and Dr. Virginia Infante, that guided me and helped me during the making of this thesis. And also thanks Diogo Rechena for the help in spell checking and proof reading. iii iv Resumo A procura de fontes energeticas´ alternativas tem crescido nos ultimos´ anos. A mudanc¸a para energia electrica´ ja´ pode ser vista na industria´ automovel,´ e a industria aeronautica´ esta´ lentamente a seguir os mesmo caminho. O uso de paineis´ solares e´ uma boa fonte energetica´ alternativa, sendo que e´ limpa e abundantemente dispon´ıvel. Esta tese foca-se num sistema que combina a gerac¸ao˜ electrica´ solar com uma propulsao˜ electrica´ numa aeronave nao˜ tripulada com 4.5 m de envergadura para missoes˜ civis de vigilanciaˆ durante longos per´ıodos de tempo. Um h´ıbrido solar-bateria e´ uma boa soluc¸ao˜ para ve´ıculos aereos´ de longa durac¸ao,˜ devido a` grande area´ dispon´ıvel nas asas para montar um painel solar capaz de fornecer energia suficiente para manter o aviao˜ no ar. -

Analysis of the Performance of an Optimization Model for Time-Shiftable Electrical Load Scheduling Under Uncertainty
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis and Dissertation Collection 2016-12 Analysis of the performance of an optimization model for time-shiftable electrical load scheduling under uncertainty Olabode, John A. Monterey, California: Naval Postgraduate School http://hdl.handle.net/10945/51591 NAVAL POSTGRADUATE SCHOOL MONTEREY, CALIFORNIA THESIS ANALYSIS OF THE PERFORMANCE OF AN OPTIMIZATION MODEL FOR TIME-SHIFTABLE ELECTRICAL LOAD SCHEDULING UNDER UNCERTAINTY by John A. Olabode December 2016 Thesis Co-Advisors: Susan M. Sanchez Emily M. Craparo Second Reader: W. Matthew Carlyle Approved for public release. Distribution is unlimited. THIS PAGE INTENTIONALLY LEFT BLANK REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704–0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instruction, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202–4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington DC 20503. 1. AGENCY USE ONLY (Leave Blank) 2. REPORT DATE 3. REPORT TYPE AND DATES COVERED December 2016 Master’s Thesis 01-04-2016 to 09-23-2016 4. TITLE AND SUBTITLE 5. FUNDING NUMBERS ANALYSIS OF THE PERFORMANCE OF AN OPTIMIZATION MODEL FOR TIME- SHIFTABLE ELECTRICAL LOAD SCHEDULING UNDER UNCERTAINTY 6. AUTHOR(S) John A. Olabode 7. -

Parametric Scramjet Analysis
University of Mississippi eGrove Electronic Theses and Dissertations Graduate School 2014 Parametric Scramjet Analysis Jongseong Choi University of Mississippi Follow this and additional works at: https://egrove.olemiss.edu/etd Part of the Aerospace Engineering Commons Recommended Citation Choi, Jongseong, "Parametric Scramjet Analysis" (2014). Electronic Theses and Dissertations. 535. https://egrove.olemiss.edu/etd/535 This Thesis is brought to you for free and open access by the Graduate School at eGrove. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of eGrove. For more information, please contact [email protected]. PARAMETRIC SCRAMJET ANALYSIS A Thesis presented in partial fulfillment of requirements for the degree of Master of Science in the Department of Mechanical Engineering The University of Mississippi by JONGSEONG CHOI May 2014 Copyright Jongseong Choi 2014 ALL RIGHT RESERVED ABSTRACT The performance of a hypersonic flight vehicle will depend on existing materials and fuels; this work presents the performance of the ideal scramjet engine for three different combustion chamber materials and three different candidate fuels. Engine performance is explored by parametric cycle analysis for the ideal scramjet as a function of material maximum service temperature and the lower heating value of jet engine fuels. The thermodynamic analysis is based on the Brayton cycle as similarly employed in describing the performance of the ramjet, turbojet, and fanjet ideal engines. The objective of this work is to explore material operating temperatures and fuel possibilities for the combustion chamber of a scramjet propulsion system to show how they relate to scramjet performance and the seven scramjet engine parameters: specific thrust, fuel-to-air ratio, thrust-specific fuel consumption, thermal efficiency, propulsive efficiency, overall efficiency, and thrust flux. -

A Parametric Analysis of a Turbofan Engine with an Auxiliary Bypass Combustion Chamber – the Turboaux Engine
Old Dominion University ODU Digital Commons Mechanical & Aerospace Engineering Theses & Dissertations Mechanical & Aerospace Engineering Fall 12-2020 A Parametric Analysis of a Turbofan Engine with an Auxiliary Bypass Combustion Chamber – The TurboAux Engine Kaleab Fetahi Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/mae_etds Part of the Mechanical Engineering Commons, Thermodynamics Commons, and the Transportation Commons Recommended Citation Fetahi, Kaleab. "A Parametric Analysis of a Turbofan Engine with an Auxiliary Bypass Combustion Chamber – The TurboAux Engine" (2020). Master of Science (MS), Thesis, Mechanical & Aerospace Engineering, Old Dominion University, DOI: 10.25777/55dp-vd74 https://digitalcommons.odu.edu/mae_etds/330 This Thesis is brought to you for free and open access by the Mechanical & Aerospace Engineering at ODU Digital Commons. It has been accepted for inclusion in Mechanical & Aerospace Engineering Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. A PARAMETRIC ANALYSIS OF A TURBOFAN ENGINE WITH AN AUXILIARY BYPASS COMBUSTION CHAMBER – THE TURBOAUX ENGINE by Kaleab Fetahi B.S. May 2019, Old Dominion University M.S. December 2020, Old Dominion University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE MECHANICAL ENGINEERING OLD DOMINION UNIVERSITY December 2020 Approved by: Sharan Asundi (Director) Arthur C. Taylor (Co-Director) Adem Ibrahim (Member) ii ABSTRACT A PARAMETRIC ANALYSIS OF A TURBOFAN ENGINE WITH AN AUXILIARY BYPASS COMBUSTION CHAMBER – THE TURBOAUX ENGINE Kaleab Fetahi Old Dominion University, 2020 Director: Dr. Sharan Asundi A parametric study of a novel turbofan engine with an auxiliary combustion chamber, nicknamed the TurboAux engine is presented. -

A Thesis Entitled Performance Analysis of J85 Turbojet Engine
A Thesis entitled Performance Analysis of J85 Turbojet Engine Matching Thrust with Reduced Inlet Pressure to the Compressor by Santosh Yarlagadda Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Mechanical Engineering Dr. Konstanty C Masiulaniec, Committee chair Dr. Terry Ng, Committee Member Dr. Sorin Cioc, Committee Member Dr. Patricia Komuniecki Dean, College of Graduate Studies The University of Toledo May 2010 Copyright 2010, Santosh Yarlagadda This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author An Abstract of Performance Analysis of J85 Turbojet Engine Matching Thrust with Reduced Inlet Pressure to the Compressor by Santosh Yarlagadda Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Mechanical Engineering The University of Toledo May 2010 Jet engines are required to operate at a higher rpm for the same thrust values in cases such as aircraft landing and military loitering. High rpm reflects higher efficiency with increased pressure ratio. This work is focused on performance analysis of a J85 turbojet engine with an inlet flow control mechanism to increase rpm for same thrust values. Developed a real-time turbojet engine integrating aerothermodynamics of engine components, principles of jet propulsion and inter component volume dynamics represented in 1-D non-linear unsteady equations. Software programs SmoothC and SmoothT were used to derive the data from characteristic rig test performance maps for the compressor and turbine respectively. Simulink, a commercially available model- based graphical block diagramming tool from MathWorks has been used for dynamic modeling of the engine. -
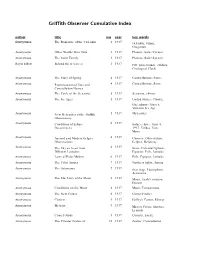
Griffith Observer Cumulative Index
Griffith Observer Cumulative Index author title mo year key words Anonymous The Romance of the Calendar 2 1937 calendar, Julian, Gregorian Anonymous Other Worlds than Ours 3 1937 Planets, Solar System Anonymous The S ola r Fa mily 3 1937 Planets, Solar System Roya l Elliott Behind the Sciences 3 1937 GO, pla ne ta rium, e xhibits , Ge ologica l Clock Anonymous The Stars of Spring 4 1937 Cons te lla tions , S ta rs , Anonymous Pronunciation of Star and 4 1937 Cons te lla tions , S ta rs Constellation Names Anonymous The Cycle of the Seasons 5 1937 Seasons, climate Anonymous The Ice Ages 5 1937 United States, Climate, Greenhouse Gases, Volcano, Ice Age Anonymous New Meteorites at the Griffith 5 1937 Meteorites Observatory Anonymous Conditions of Eclipse 6 1937 Solar eclipse, June 8, Occurrences 1937, Umbra, Sun, Moon Anonymous Ancient and Modern Eclipse 6 1937 Chinese, Observation, Observations Eclips e , Re la tivity Anonymous The Sky as Seen from 6 1937 Stars, Celestial Sphere, Different Latitudes Equator, Pole, Latitude Anonymous Laws of Polar Motion 6 1937 Pole, Equator, Latitude Anonymous The Polar Aurora 7 1937 Northern lights, Aurora Anonymous The Astrorama 7 1937 Star map, Planisphere, Astrorama Anonymous The Life Story of the Moon 8 1937 Moon, Earth's rotation, Darwin Anonymous Conditions on the Moon 8 1937 Moon, Temperature, Anonymous The New Comet 8 1937 Come t Fins le r Anonymous Comets 9 1937 Halley's Comet, Meteor Anonymous Meteors 9 1937 Meteor Crater, Shower, Leonids Anonymous Comet Orbits 9 1937 Comets, Encke Anonymous -

Estimating Cell Capacity for Multi-Cell Electrical Energy System
Dissertations and Theses Spring 2013 Estimating Cell Capacity for Multi-Cell Electrical Energy System Iman Ahari Hashemi Embry-Riddle Aeronautical University - Daytona Beach Follow this and additional works at: https://commons.erau.edu/edt Part of the Computer Engineering Commons, and the Electrical and Electronics Commons Scholarly Commons Citation Hashemi, Iman Ahari, "Estimating Cell Capacity for Multi-Cell Electrical Energy System" (2013). Dissertations and Theses. 80. https://commons.erau.edu/edt/80 This Thesis - Open Access is brought to you for free and open access by Scholarly Commons. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. Estimating Cell Capacity for Multi-Cell Electrical Energy System by Iman Ahari Hashemi A Thesis Submitted to the College of Engineering, Department of Electrical, Computer, Software & Systems Engineering In Partial Fulfillment of the Requirements for the Degree of Master of Science in Electrical and Computer Engineering Embry-Riddle Aeronautical University Daytona Beach, Florida Spring 2013 Acknowledgements I would like to give special thanks to the Committee Chair Dr. Jianhua Liu for his invaluable guidance throughout the time that research was conducted. I would also like to thank the supervision of the committee members Dr. Ilteris Demirkiran and Dr. Thomas Yang. It would be of great honor to thank the endless loving support which I received from my family back home throughout my course of studies. iii Abstract Researcher: Iman Ahari Hashemi Title: Estimating Cell Capacity for Multi-Cell Electrical Energy System Institution: Embry-Riddle Aeronautical University Degree: Master of Science in Electrical and Computer Engineering Year: 2013 A Multi-Cell Electrical Energy System is a set of batteries that are connected in series.