2020 MAPD Formulary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
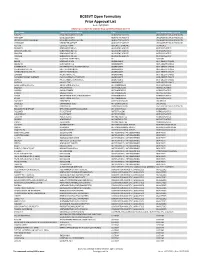
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED)
Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED) A Abatacept (ORENCIA) Abemaciclib (VERZENIO) Abiraterone Acetate (ZYTIGA) Abiraterone Acetate (+ GENERIC FORMULATIONS) AbobotulinumtoxinA (DYSPORT) Abraxane (ABRAXANE) Acalabrutinib (CALQUENCE) Actemra (ACTEMRA) Adalimumab (AMGEVITA) Adalimumab (HADLIMA) Adalimumab (HULIO) Adalimumab (HUMIRA) Adalimumab (HYRIMOZ) Adalimumab (IDACIO) Adcetris (ADCETRIS) Adcirca (ADCIRCA) Adempas (ADEMPAS) Afatinib (GIOTRIF) Afinitor (AFINITOR) Aflibercept (EYLEA) Aflibercept (ZALTRAP) Afstyla (AFSTYLA) Agalsidase Alfa (REPLAGAL) Agalsidase Beta (FABRAZYM) Aimovig (AIMOVIG) Ajovy (FREMANEZUMAB) Aldesleukin (PROLEUKIN) Aldurazyme (ALDURAZYME) Alecensaro (ALECENSARO) Alectinib (ALECENSARO) Alefacept (AMEVIVE) Alemtuzumab (MABCAMPATH) Alemtuzumab (LEMTRADA) Alglucosidase Alfa (MYOZYME) Alimta (ALIMTA) Alirocumab (PRALUENT) Alpha-1-Proteinase Inhibitor (PROLASTIN - C) Alpha-1-Proteinase Inhibitor (ZEMAIRA) Alpelisib (PIQRAY) Alunbrig (ALUNBRIG) Ambrisentan (VOLIBRIS) Ambrisentan (Generic Formulations) Amevive (AMEVIVE) Amgevita (ADALIMUMAB) Amifampridine Phosphate (FIRDAPSE) Amifampridine Phosphate (RUZURGI) Anakinra (KINERET) Apalutamide (ERLEADA) Apomorphine (KYNMOBI) Apremilast (OTEZLA) Page 1 of 17 Updated: May 20, 2021 Arsenic Trioxide (TRISENOX) Arsenic Trioxide (Generic Formulations) Arzerra (ARZERRA) Asfotase Alfa (STRENSIQ) Asparaginase (ERWINASE) Asunaprevir (SUNVEPRA) Atezolizumab (TECENTRIQ) Atriance (ATRIANCE) Aubagio (AUBAGIO) Avastin (AVASTIN) Avelumab (BAVENCIO) Avonex -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

761094Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761094Orig1s000 CLINICAL REVIEW(S) Medical Officer’s Review of BLA 761094 Review #2 BLA 761094 Submission Date: July 19, 2018 Receipt Date: July 19, 2018 Review Date: July 23, 2018 Applicant: Dompé farmaceutici S.p.A. Via Santa Lucia 6-20122 Milan, Italy Applicant’s Representative: Lamberto Dionigi. Regulatory Affairs & Drug Safety Director 39-02-5838-3559 Drug: OXERVATE (cenegermin – bkbj) ophthalmic solution, 20 mcg/mL Submitted: Reference is made to the meeting Teleconference of 15-July-2018, in which the FDA has requested to provide information available at Dompé on the use of Oxervate in the pediatric population. Pediatric patients exposed to Oxervate: Dompé confirms that Oxervate is not yet approved in Europe for use in children and no clinical studies have been conducted in this population. As far as the company is aware, four (4) Neurotrophic Keratitis (NK) pediatric patients, aged from 2 to 12 years of age, have been exposed to Oxervate. A 6-year-old child with severe NK secondary to Stuve-Wiedmann syndrome was treated with Oxervate under “Temporary Authorization for Use” (ATU) in France. Dompé reports that the corneal ulcer was completely healed at the end of the treatment. There were signs of corneal sensitivity recovery by month 8 post-treatment. A 9-year-old child with NK was treated with Oxervate under ATU in France. Dompé reports that the lesion improved, but did not completely heal with the treatment. There is no other follow-up information available. A 12-year-old child with severe NK was treated with Oxervate under Expanded Access in the US. -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England V14 Changes to Version 13
MEDICINES NOT REIMBURSED THROUGH NATIONAL PRICES AND DIRECTLY COMMISSIONED BY NHS ENGLAND V14 CHANGES TO VERSION 13 PUBLISHED APRIL 2019 Changes to v13 Lines removed SUITABLE SPECIALIST FOR CENTRE SUITABLE FOR SHARED ONLY SHARED CARE CARE PRIOR (includng WITH PRIMARY BETWEEN STOPPING MONITORING/ AUDIT APPROVAL outreach CARE (IF DRUG NAME INDICATION COMMISSIONER PBR CATEGORY TA/POLICY STARTING CRITERIA SPECIALIST COMMENT CRITERIA REQUIREMENTS PROFORMA when SUPPORTED AND REQUIRED delivered as BY LOCAL SECONDARY part of a PRESCRIBING CARE VIA provider COMMITTEE) NETWORK network) MODEL PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER ADULT TA'S (TA195, TA373, ABATACEPT NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE √ MEDICINES FOR CHILDREN TA375) POLICY) Now covered by comment 8 PAEDIATRIC INDICATIONS (IN AS PER TA455 OR ADULT TA'S (TA130 LINE WITH NHS ENGLAND (replaced by TA375), TA143 (repalced by ADALIMUMAB NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE AUDIT √ √ MEDICINES FOR CHILDREN TA383), TA146, TA187, TA199, TA329, POLICY) TA392, TA460) Now covered by comment 8 ALBUTREPENONACOG ALFA HAEMOPHILIA B PRODUCTS ON CMU Blood factor products simplified NHS ENGLAND BLOOD-RELATED PRODUCTS SSC 1652 SSC 1652 SSC 1652 √ FRAMEWORK to blood factor product VIII etc PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER IFR AS PER IFR ANAKINRA NHS ENGLAND CYTOKINE MODULATORS NOT ROUTINELY COMMISSIONED AS PER IFR APPROVAL √ MEDICINES FOR CHILDREN APPROVAL APPROVAL POLICY) Now covered by comment 8 AS PER BCSH GUIDELINES ANTIHAEMOPHILIC FACTOR/VON -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Pegvaliase-Pqpz
Pharmacy Benefit Coverage Criteria Effective Date………….………….....................12/1/2020 Next Review Date………………………………. 12/1/2021 Coverage Policy Number ................................ P0055 Pegvaliase-pqpz Table of Contents Related Coverage Resources Medical Necessity Criteria ................................... 1 Sapropterin FDA Approved Indications ................................... 2 Recommended Dosing ........................................ 2 General Background ............................................ 3 References .......................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s -

Specialty Pharmacy Program Drug List
Specialty Pharmacy Program Drug List The Specialty Pharmacy Program covers certain drugs commonly referred to as high-cost Specialty Drugs. To receive in- network benefits/coverage for these drugs, these drugs must be dispensed through a select group of contracted specialty pharmacies or your medical provider. Please call the BCBSLA Customer Service number on the back of your member ID card for information about our contracted specialty pharmacies. All specialty drugs listed below are limited to the retail day supply listed in your benefit plan (typically a 30-day supply). As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. Please refer to your benefit plan for a complete list of benefits, including specific exclusions, limitations and member cost-sharing amounts you are responsible for such as a deductible, copayment and coinsurance. Brand Name Generic Name Drug Classification 8-MOP methoxsalen Psoralen ACTEMRA SC tocilizumab Monoclonal Antibody/Arthritis ACTHAR corticotropin Adrenocortical Insufficiency ACTIMMUNE interferon gamma 1b Interferon ADCIRCA tadalafil Pulmonary Vasodilator ADEMPAS riociguat Pulmonary Vasodilator AFINITOR everolimus Oncology ALECENSA alectinib Oncology ALKERAN (oral) melphalan Oncology ALUNBRIG brigatinib Oncology AMPYRA ER dalfampridine Multiple Sclerosis APTIVUS tipranavir HIV/AIDS APOKYN apomorphine Parkinson's Disease ARCALYST rilonacept Interleukin Blocker/CAPS ATRIPLA efavirenz-emtricitabine-tenofovir HIV/AIDS AUBAGIO -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -

PALYNZIQ (Pegvaliase-Pqpz) Injection, for Subcutaneous Use Blood Phenylalanine Monitoring and Diet (2.2) Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION 600 micromol/L after 16 weeks of continuous treatment with the These highlights do not include all the information needed to use maximum dosage of 40 mg once daily. PALYNZIQ safely and effectively. See full prescribing information for Reduce the dosage and/or modify dietary protein and phenylalanine PALYNZIQ. intake, as needed, to maintain blood phenylalanine concentrations within a clinically acceptable range and above 30 micromol/L. PALYNZIQ (pegvaliase-pqpz) injection, for subcutaneous use Blood Phenylalanine Monitoring and Diet (2.2) Initial U.S. Approval: 2018 Obtain blood phenylalanine concentrations every 4 weeks until a maintenance dosage is established. WARNING: RISK OF ANAPHYLAXIS After a maintenance dosage is established, periodically monitor blood See full prescribing information for complete boxed warning. phenylalanine concentrations. Anaphylaxis has been reported after administration of Palynziq Counsel patients to monitor dietary protein and phenylalanine intake, and may occur at any time during treatment. (5.1) and adjust as directed by their healthcare provider. Administer the initial dose of Palynziq under the supervision of a Premedication (2.3, 5.1, 5.3) healthcare provider equipped to manage anaphylaxis, and closely Consider premedication for hypersensitivity reactions. observe patients for at least 60 minutes following injection. Prior Administration Instructions (2.4) to self-injection, confirm patient competency with Rotate injection sites. If more than one injection is needed for a single self-administration, and patient’s and observer’s (if applicable) dose, the injection sites should be at least 2 inches away from each other. ability to recognize signs and symptoms of anaphylaxis and to administer auto-injectable epinephrine, if needed.