New Mineral Names*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1457 Vol 43#5 Art 02.Indd
1457 The Canadian Mineralogist Vol. 43, pp. 1457-1468 (2005) WILUITE FROM ARICCIA, LATIUM, ITALY: OCCURRENCE AND CRYSTAL STRUCTURE FABIO BELLATRECCIA Dipartimento di Scienze della Terra, Università di Roma “La Sapienza”, Piazzale Aldo Moro 5, I–00185 Roma, Italy, and Dipartimento di Scienze Geologiche, Università Roma Tre, Largo S. Leonardo Murialdo 1, I–00146 Roma, Italy FERNANDO CÁMARA AND LUISA OTTOLINI CNR – Istituto di Geoscienze e Georisorse, Sede di Pavia, via Ferrata 1, I–27100 Pavia, Italy GIANCARLO DELLA VENTURA§, GIANNANTONIO CIBIN AND ANNIBALE MOTTANA Dipartimento di Scienze Geologiche, Università Roma Tre, Largo S. Leonardo Murialdo 1, I–00146 Roma, Italy ABSTRACT We report a new occurrence of the rare mineral wiluite, the B-rich equivalent of vesuvianite, from Ariccia, Alban Hills volcano, Rome, Italy. The specimen studied was found in the collection of the Museum of Mineralogy of the University of Rome (label MMUR22496/482). Wiluite from Ariccia was sampled at the Parco Chigi quarry, within the phreatomagmatic unit emitted by the Albano maar known locally as “Peperino di Marino”. It occurs as dark brown to black euhedral, well-formed prismatic crystals, up to 1.0 cm in length and 0.5 cm across. Optical observations show a weak pleochroism and an imperfect extinction. The mineral is uniaxial (+) with 1.722(2) and 1.727(2). It is tetragonal P4/nnc, a 15.716(2), c 11.704(2) Å, V 2890.8(7) Å3. The crystal-chemical formula, obtained by combining EMP, SIMS and XREF data and calculated on the basis of 18 Si atoms, is: X Y(1) 3+ Y(2) 3+ Y(3) 3+ T(1) (Ca18.72Mg0.15Sr0.02La0.05Ce0.04Nd0.01) (Fe 0.36Mg0.26Ti0.27Mn0.11) (Al3.67Fe 0.19Mg0.14) (Al3.29Fe 1.36Mg3.35) (B2.18 T(2) Z O(11) [O(10)+O(12)] Al0.02Be0.02H0.47Ⅺ1.31) (B0.68H0.32) Si18O68 (F1.21O6.79) O2.68. -

Relationship Among Metamorphic Grade, Vesuvianite “Rod Polytypism,” and Vesuvianite Composition
American Mineralogist, Volume 91, pages 862–870, 2006 Relationship among metamorphic grade, vesuvianite “rod polytypism,” and vesuvianite composition EDWIN GNOS1,* AND THOMAS ARMBRUSTER2 1Institut für Geologie, Universität Bern, Baltzerstrasse 1-3, CH-3012 Bern, Switzerland 2Laboratorium für chemische und mineralogische Kristallographie, Universität Bern, Freiestrasse 3, CH-3012 Bern, Switzerland ABSTRACT Single-crystal X-ray study of different vesuvianite samples of known origin shows that differ- ent metamorphic grade results in different arrangements of structural rods oriented parallel to the vesuvianite c axis, interpreted as “rod polytypism.” There is a systematic dependence of space-group symmetry and rod arrangement on crystallization temperature: P4nc-dominant < 300 °C, P4/n-domi- nant ~300–500 °C, and P4/nnc > 500 °C. Partial occupancy of the T sites (B, Al, Fe3+) and increased F-content seem to stabilize rod disorder causing P4/nnc space-group symmetry. All studied vesuvianites in calcsilicate rocks and marbles from regional- and contact-metamorphic upper amphibolite facies have disordered rods (P4/nnc symmetry). Electron-microprobe analyses of metamorphic vesuvianites from alpine and non-alpine occurrences, supported by structural investigation, showed that in addi- tion to homo- and heterovalent substitution, partial occupancy of the commonly vacant T sites by B, 3+ 4– → 4– Al, or Fe , and the (O4H4) SiO4 (hydrogarnet-type) substitutions are signiÞ cant in nature. With few exceptions, T-site occupancy seems to be restricted to high-grade metamorphic rocks whereas the “hydrovesuvianite” substitution is only found in vesuvianites formed at very low metamorphic grade. The cell parameters of vesuvianite with empty T sites increase with increasing Ti + Mg → 2 Al substitution, and this increase is even more pronounced with increasing “hydrovesuvianite” component. -

Nomenclature of the Garnet Supergroup
American Mineralogist, Volume 98, pages 785–811, 2013 IMA REPORT Nomenclature of the garnet supergroup EDWARD S. GREW,1,* ANDREW J. LOCOCK,2 STUART J. MILLS,3,† IRINA O. GALUSKINA,4 EVGENY V. GALUSKIN,4 AND ULF HÅLENIUS5 1School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. 2Department of Earth and Atmospheric Sciences, University of Alberta, Edmonton, Alberta T6G 2E3, Canada 3Geosciences, Museum Victoria, GPO Box 666, Melbourne 3001, Victoria, Australia 4Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, University of Silesia, Będzińska 60, 41-200 Sosnowiec, Poland 5Swedish Museum of Natural History, Department of Mineralogy, P.O. Box 50 007, 104 05 Stockholm, Sweden ABSTRACT The garnet supergroup includes all minerals isostructural with garnet regardless of what elements occupy the four atomic sites, i.e., the supergroup includes several chemical classes. There are pres- ently 32 approved species, with an additional 5 possible species needing further study to be approved. The general formula for the garnet supergroup minerals is {X3}[Y2](Z3)ϕ12, where X, Y, and Z refer to dodecahedral, octahedral, and tetrahedral sites, respectively, and ϕ is O, OH, or F. Most garnets are cubic, space group Ia3d (no. 230), but two OH-bearing species (henritermierite and holtstamite) have tetragonal symmetry, space group, I41/acd (no. 142), and their X, Z, and ϕ sites are split into more symmetrically unique atomic positions. Total charge at the Z site and symmetry are criteria for distinguishing groups, whereas the dominant-constituent and dominant-valency rules are critical in identifying species. Twenty-nine species belong to one of five groups: the tetragonal henritermierite group and the isometric bitikleite, schorlomite, garnet, and berzeliite groups with a total charge at Z of 8 (silicate), 9 (oxide), 10 (silicate), 12 (silicate), and 15 (vanadate, arsenate), respectively. -

4358 Revision 2 1 Fe-Rich and As-Bearing Vesuvianite and Wiluite
4358 Revision 2 1 Fe-rich and As-bearing vesuvianite and wiluite from Kozlov, Czech Republic 2 ∗ 3 LEE A. GROAT,1, R. JAMES EVANS,1 JAN CEMPÍREK,1 CATHERINE McCAMMON2, 4 AND STANISLAV HOUZAR3 5 6 1Department of Earth, Ocean and Atmospheric Sciences, University of British Columbia, 7 Vancouver, British Columbia V6T 1Z4, Canada 8 9 2Bayerisches Geoinstitut, Universität Bayreuth, D-95440 Bayreuth, Germany 10 11 3Department of Mineralogy and Petrography, Moravian Museum, Zelný trh 6, 659 37 Brno, 12 Czech Republic 13 ∗ ∗E-mail: [email protected] 1 4358 Revision 2 14 ABSTRACT 15 16 Green vesuvianite crystals occur with garnet and calcite in a hand specimen from the 17 Nedvědice marble near Kozlov (near Štěpánov nad Svratkou, Svratka Crystalline Complex) in 18 the Czech Republic. The average electron microprobe composition of the vesuvianite shows 19 12.10 wt.% Fe2O3 (4.66 Fe pfu), 2.77 wt.% B2O3 (2.45 B pfu), 1.71 wt.% As2O5 (0.46 As pfu), 20 and 1.40 wt.% F (2.26 F pfu). The Fe concentration is the highest ever recorded for a 21 vesuvianite-group mineral. The boron contents are extremely variable and two of the five 22 compositions show more than the 2.50 B pfu needed for wiluite, and the average is only slightly 23 less than this. The crystal structure [a = 15.7250(4), c = 11.7736(3) Å] was refined in space 24 group P4/nnc to an R1 value of 0.0221. The site refinement and Mössbauer spectroscopy results 25 show Fe2+ substituting for Ca at the X3 site and filling the Y1 position, and Fe3+ substituting for 26 Al at the Y3 position. -

Irina GALUSKINA1, Evgeny GALUSKIN1, Roman W£ODYKA1, Piotr DZIER¯ANOWSKI2, Roman WRZALIK3
MINERALOGIA POLONICA DOI 10.2478/v10002-007-0022-9 PL ISSN 0032-6267 Vol. 38, No 2, 2007 Irina GALUSKINA1, Evgeny GALUSKIN1, Roman W£ODYKA1, Piotr DZIER¯ANOWSKI2, Roman WRZALIK3 ATOLL GARNETS IN “ACHTARANDITE” SERPENTINITES: MORPHOLOGY, COMPOSITION AND MODE OF ORIGIN Received April 26, 2007; accepted November 14, 2007 A b s t r a c t . Atoll garnets in aposkarn serpentinite from the Wiluy River, Republic of Sakha-Yakutia, Russia, have the classic form comprising a garnet core, an intermediate zone filled with chlorite-group minerals and an outer garnet atoll. The core of an illustrated example is complexly zoned from schorlomite to grossular-andradite. Morphologically, the core is a rhombic dodecahedral crystal. The atoll crystallized as a tetragon-trisoctahedron with minor rhombic dodecahedron faces and is composed of hibschite and “hydroandradite”. The atoll garnet formed as the result of selective dissolution and substitution by chlorite of an internal hibschite zone with columnar structure that became unstable under new conditions of crystallization. The pattern of dissolution traces defects in the garnet crystal. The growth of the atoll garnets reflects the main stages in the evolution of the Wiluy deposit itself and is associated with the development of the Siberian traps. Key-words: atoll garnet, morphology, hibschite, Raman spectroscopy, serpentinite, Wiluy River, Russia INTRODUCTION Beginning twelve years ago, during work on “achtarandite” represented by hib- schite pseudomorphs of wadalite (Galuskin et al. 1995; Galuskina et al. 1998), we encountered minerals of the hydrogarnet group for the first time. Searching the lite- rature on hydrogarnet, we discovered the valuable early work on crystal chemistry of hydrogarnets published by Professor Witold ¯abiñski in 1965 (¯abiñski 1965a, b). -

Si-DEFICIENT, OH-SUBSTITUTED, BORON-BEARING VESUVIANITE from the WILUY RIVER, YAKUTIA, RUSSIA
833 Volume 41 August 2003 Part 4 The Canadian Mineralogist Vol. 41, pp. 833-842 (2003) Si-DEFICIENT, OH-SUBSTITUTED, BORON-BEARING VESUVIANITE FROM THE WILUY RIVER, YAKUTIA, RUSSIA EVGENY V. GALUSKIN§ AND IRINA O. GALUSKINA§ Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, University of Silesia, Be¸dzi´nska 60, 41–200 Sosnowiec, Poland MACIEJ SITARZ Department of Material Sciences and Ceramics, University of Mining and Metallurgy, al. Mickiewicza 30, Cracow, 30–059, Poland KATARZYNA STADNICKA Faculty of Chemistry, Jagellonian University, Ingardena 3, Cracow, 30–060, Poland ABSTRACT A low-temperature, Si-deficient variety of vesuvianite occurs in porous tetrahedral “achtarandite” pseudomorphs consisting 4– of hibschite, along the banks of the Wiluy River, Yakutia, Russia, the type locality of grossular and wiluite. The (H4O4) -for- 4– (SiO4) hydrogarnet-type substitution is evident in the vesuvianite, a substitution that allows it to be considered an analogue of hibschite. This variety of vesuvianite belongs to a new series in the vesuvianite group, as expressed by the formula X19Y13T0–5(Si2O7)4(SiO4)10–x(OH)4xW10. The filling of the X, Y, and T positions in this Si-deficient vesuvianite, where x varies from 0.67 to 2.89, is analogous to that in vesuvianite and wiluite. The Si-deficient vesuvianite is characterized by increased unit- cell parameters, a 15.688(3), c 11.860(3) Å and by lower indices of refraction, 1.691(1), 1.668(1). In the OH-region, the FTIR and Raman spectra differ sharply from those of low-temperature vesuvianite from rodingites, but are similar to the spectra of hibschite. -

Subject Index, Volume 84, 1999
American Mineralogist, Volume 84, pages 1985–1992, 1999 SUBJECT INDEX, VOLUME 84, 1999 Aenigmatite 257 andalusite 1727 ites and related rocks. By A.F. Aerinite 1467 Annealing 1213, 1224, 1235 Glazner 1210 AFM (SFM, STM) 620, 884 Anion-centered tetrahedra 1099 Coppens, P.: X-Ray Charge hematite 1061 Anomalous scattering 294 Densities and Chemical Bonding. ilmenite 1384 Arnhemite 193 By J.W.Downs 690 pyrite 1549 Apatite 581, 977, 1213, 1224, 1235, Dyar, M.D., McCammon, C. Akimotite 226, 233, 267 1346 and Schaefer M.W.: Mineral Al2SiO5 group 1152 dental 1406 Spectroscopy: A Tribute to Roger Albite 27, 333, 726, 746, 1144 Armenite 86, 92 G. Burns. By A. Treiman 211 melting 1830 Arsenides 639 Grew, E.S. and Anovitz, L.M.: ALH84001 1569 Ashanite 688 Boron: Mineralogy, Petrology Alkali basalt 357 Au, As in pyrite 1071 and Geochemistry. By E.E Allanite 1346 Auorthosites 806 Foord, J.T. O’Connor 1209 Almandine 374 Au2SbO2(OH) 197 Hughes, R.W.: Ruby and Sapphire. AlO6 octahedral chains 1152 Averievite 685 By J. Sinkankas 211 Aluminosilicates 152, 311, 345, 465, Awards Moore, D.M. and Reynolds, R. C., Jr.: 983 Distinguished Public Service X-ray Diffraction and the Identifi- Alunite-group minerals 1687 Medal, acceptance of 1207 cation and Analysis of Clay Miner- Amphibole 1, 86, 102, 1033, 1304 Distinguished Public Service als, second edition. By S.P. Altaner, Analcime 112 Medal, presentation of 1205 689 Analysis chem. (mineral) 1170 Mineralogical Society of America, Young, D.A.: N.L. Bowen and aenigmatite 257 acceptance of 1203 cystallization—differentation: amphibole 102 Mineralogical Society of America, The evolution of a theory.By bederite 1674 presentation of 1202 H.S. -

THE CRYSTAL STRUCTURE of Si-DEFICIENT, OH-SUBSTITUTED, BORON-BEARING VESUVIANITE from the WILUY RIVER, SAKHA-YAKUTIA, RUSSIA
239 The Canadian Mineralogist Vol. 45, pp. 239-248 (2007) THE CRYSTAL STRUCTURE OF Si-DEFICIENT, OH-SUBSTITUTED, BORON-BEARING VESUVIANITE FROM THE WILUY RIVER, SAKHA-YAKUTIA, RUSSIA Evgeny V. GALUSKIN§ and Irina O. GALUSKINA Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, University of Silesia, Będzińska 60, 41–200 Sosnowiec, Poland Katarzyna STADNICKA Faculty of Chemistry, Jagiellonian University, Ingardena 3, Cracow, 30–060, Poland Thomas ARMBRUSTER Laboratory for Chemical and Mineralogical Crystallography, University of Bern, Freiestr. 3, CH–3012 Bern, Switzerland Marcin KOZANECKI Technical University, Department of Molecular Physics, Żeromskiego 116, 90–924 Łódż, Poland Abstract The crystal structure of a Si-defi cient vesuvianite, space group P4/nnc, a 15.678(1), c 11.828(1) Å, from the Wiluy River, Sakha–Yakutia, Russia, has been refi ned from single-crystal X-ray data to R = 0.037. Electron-microprobe analyses indicate that this vesuvianite has only ca. 16 Si pfu in contrast to regular vesuvianite with 18 Si pfu. Site-occupancy refi nement yielded substantial vacancies at orthosilicate sites Z(1): 25% vacancies, Z(2): 16% vacancies. Vacancies at the tetrahedral site are associated with increased Z(1)–O and Z(2)–O distances, 1.687 and 1.660 Å, respectively. Vacancies and increased Z(1)–O and Z(2)–O bond lengths are consistent with hydrogarnet-type defects, where SiO4 is replaced by H4O4 tetrahedra. The single crystal investigated shows the highest hydrogarnet-type substitution analyzed by structure refi nement of vesuvianite. No vacancies were found involving the disilicate groups. Along the c axis, the increased size of Z(1) tetrahedra is balanced by a compression of the adjacent X(3) Ca-bearing dodecahedra. -

Mineralogical Notes Sekies 1
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 490 MINERALOGICAL NOTES SEKIES 1 BY WALDEMAR T. SCHALLER WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Page. Introduction............................................................. 7 Chemical composition of hulsite and paigeite...........................__ 8 Introduction.....................................................__ 8 Occurrence and association........................................_. 8 Notes on chemical examination...................__............_. 10 General statement...........................__................. 10 The gangue...................................................... 11 Methods of analyses.............................................. 13 Hulsite.............................................................. 16 Crystallography............... ... ....... ................ 16 General properties........ ................. ............... 16 Character of samples ............................................. 17 Analyses and ratios.......................__.................... 18 Discussion of formulas............................................ 20 Paigeite ................................ .......................... 21 General description .............................................. 21 Character of samples............................................. 22 Analyses and ratios .............................................. 22 Discussion of formulas...... ................................. 24 Chemical composition of jamesonite -
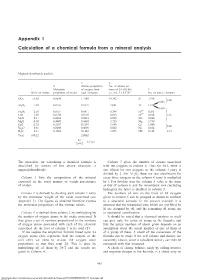
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Glossary of Obsolete Mineral Names
vaalerts = tetrahedrite, Zirlin 108 (1981). vaal-garin = pale-blue fibrous riebeckite, Thrush 1193 (1968). vaalite = vermiculite, Dana 6th, 667 (1892). vabanite = red massive Fe-rich quartz, MM 39, 929 (1974). vad = wad (pyrolusite ± manganite ± romanèchite ± cryptomelane), László 284 (1995). vaeyrynenite = väyrynenite, Nickel & Nichols 250 (1991). vagdaltkvarc = quartz pseudomorph after baryte, László 153 (1995). vagearsite = germanocolusite, Pekov 91 (1998). vaidûrya = beryl, Bukanov 64 (2006). vairakit = wairakite, László 318 (1995). vairauit = wairauite, László 318 (1995). vajra = diamond, Bukanov 39 (2006). vakabajasilit = wakabayashilite, László 318 (1995). valahite = illite-smectite mixed-layer, MA 17, 138 (1965). Valait = bitumen, Dana 6th, 1051 (1892). valchovite = resin (C15H26O)n, Clark 729 (1993). Valencianit = orthoclase, Dana 6th, 315 (1892). valentianite = orthoclase, Chester 280 (1896). valeriite = valleriite, Dana 6th I, 71 (1899). valhovit = resin, László 318 (1995). vallachite = illite-smectite mixed-layer, MM 35, 1158 (1966); 38, 103 (1971). valléite = Ca-Mn-rich anthophyllite, AM 63, 1052 (1978). Vallendar Clay = kaolinite ?, Robertson 33 (1954). valleriite-(Fe) = FeCuS.1·5Fe(OH)2, AM 57, 1051 (1972). valleriite-(Mg,Al) = valleriite, AM 57, 1051 (1972). valleriite-(Mg,Fe) = haapalaite, AM 57, 1051 (1972). valleriite type II = tochilinite, AM 59, 190 (1974). vallerite = valleriite, R. Dixon. pers. comm. (1992). valley brown ore = goethite, Thrush 1195 (1968). Vallumdiamant = transparent quartz, Haditsch & Maus 229 (1974). vallum diamond = transparent quartz, AM 12, 385 (1927). vallum stone = transparent quartz, AM 12, 386 (1927). valpurgit = walpurgite, László 319 (1995). valuevite = Al-rich clintonite, AM 52, 1122 (1967). valujevit = Al-rich clintonite, László 284 (1995). vamaite = resin (C11H16O2 ?), Clark 730 (1993). vanadanite = vanadinite, Embrey & Fuller 173 (1980). vanadate of copper = volborthite, Dana 6th, 838 (1892). -

"Rod" Polytypism in Vesuvianite : Crystal Structure of a Low-Temperatur P4nc Vesuvianite with Pronounced Octahedral Cation Ordering
"Rod" polytypism in vesuvianite : crystal structure of a low-temperatur P4nc vesuvianite with pronounced octahedral cation ordering Autor(en): Armbruster, Thomas / Gnos, Edwin Objekttyp: Article Zeitschrift: Schweizerische mineralogische und petrographische Mitteilungen = Bulletin suisse de minéralogie et pétrographie Band (Jahr): 80 (2000) Heft 2 PDF erstellt am: 26.09.2021 Persistenter Link: http://doi.org/10.5169/seals-60955 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot