Pathways of Clathrin-Independent Endocytosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

An Arf1 Synthetic Lethal Screen Identifies a New Clathrin Heavy
Copyright 1998 by the Genetics Society of America An arf1D Synthetic Lethal Screen Identi®es a New Clathrin Heavy Chain Conditional Allele That Perturbs Vacuolar Protein Transport in Saccharomyces cerevisiae Chih-Ying Chen and Todd R. Graham Department of Molecular Biology, Vanderbilt University, Nashville, Tennessee 37235 Manuscript received March 5, 1998 Accepted for publication June 16, 1998 ABSTRACT ADP-ribosylation factor (ARF) is a small GTP-binding protein that is thought to regulate the assembly of coat proteins on transport vesicles. To identify factors that functionally interact with ARF, we have performed a genetic screen in Saccharomyces cerevisiae for mutations that exhibit synthetic lethality with an arf1D allele and de®ned seven genes by complementation tests (SWA1-7 for synthetically lethal with arf1D). Most of the swa mutants exhibit phenotypes comparable to arf1D mutants such as temperature-conditional growth, hypersensitivity to ¯uoride ions, and partial protein transport and glycosylation defects. Here, we report that swa5-1 is a new temperature-sensitive allele of the clathrin heavy chain gene (chc1-5), which carries a frameshift mutation near the 39 end of the CHC1 open reading frame. This genetic interaction between arf1 and chc1 provides in vivo evidence for a role for ARF in clathrin coat assembly. Surprisingly, strains harboring chc1-5 exhibited a signi®cant defect in transport of carboxypeptidase Y or carboxypepti- dase S to the vacuole that was not observed in other chc1 ts mutants. The kinetics of invertase secretion or transport of alkaline phosphatase to the vacuole were not signi®cantly affected in the chc1-5 mutant, further implicating clathrin speci®cally in the Golgi to vacuole transport pathway for carboxypeptidase Y. -

ADP-Ribosylation Factor, a Small GTP-Binding Protein, Is Required for Binding of the Coatomer Protein Fl-COP to Golgi Membranes JULIE G
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6408-6412, July 1992 Biochemistry ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein fl-COP to Golgi membranes JULIE G. DONALDSON*, DAN CASSEL*t, RICHARD A. KAHN*, AND RICHARD D. KLAUSNER* *Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development, and tLaboratory of Biological Chemistry, Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892 Communicated by Marc Kirschner, April 20, 1992 (receivedfor review February 11, 1992) ABSTRACT The coatomer is a cytosolic protein complex localized to the Golgi complex, although their functions have that reversibly associates with Golgi membranes and is Impli- not been defined. Distinct among these proteins is the ADP- cated in modulating Golgi membrane transport. The associa- ribosylation factor (ARF), originally identified as a cofactor tion of 13-COP, a component of coatomer, with Golgi mem- required for in vitro cholera toxin-catalyzed ADP- branes is enhanced by guanosine 5'-[v-thioltriphosphate ribosylation of the a subunit of the trimeric GTP-binding (GTP[yS]), a nonhydrolyzable analogue of GTP, and by a protein G, (G,.) (19). ARF is an abundant cytosolic protein mixture of aluminum and fluoride ions (Al/F). Here we show that reversibly associates with Golgi membranes (20, 21). that the ADP-ribosylation factor (ARF) is required for the ARF has been shown to be present on Golgi coated vesicles binding of (-COP. Thus, 13-COP contained in a coatomer generated in the presence of GTP[yS], but it is not a com- fraction that has been resolved from ARF does not bind to Golgi ponent of the cytosolic coatomer (22). -

Palmitoylation: Implications for Nitric Oxide Signaling
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6448-6453, June 1996 Cell Biology Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling (endothelial nitric oxide synthase/signal transduction/vascular biology/N-myristoylation) GUILLERMO GARC1A-CARDENA*, PHIL OHt, JIANwEI LIu*, JAN E. SCHNITZERt, AND WILLIAM C. SESSA*t *Molecular Cardiobiology Program and Department of Pharmacology, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536; and tDepartment of Pathology, Harvard Medical School, Beth Israel Hospital, 330 Brookline Avenue, Boston, MA 02215 Communicated by Vincent T. Marchesi, Yale Univeristy, New Haven, CT, March 13, 1996 (received for review February 5, 1996) ABSTRACT The membrane association of endothelial insoluble membranes (TIM), suggesting that caveolae are nitric oxide synthase (eNOS) plays an important role in the signal processing centers (2-11). Additionally, caveolae have biosynthesis of nitric oxide (NO) in vascular endothelium. been implicated in other important cellular functions, includ- Previously, we have shown that in cultured endothelial cells ing endocytosis, potocytosis, and transcytosis (12, 13). and in intact blood vessels, eNOS is found primarily in the Endothelial nitric oxide synthase (eNOS) is a peripheral perinuclear region of the cells and in discrete regions of the membrane protein that metabolizes L-arginine to nitric oxide plasma membrane, suggesting trafficking of the protein from (NO). NO is a short-lived free radical gas involved in diverse the Golgi to specialized plasma membrane structures. Here, physiological and pathological processes. Endothelial-derived we show that eNOS is found in Triton X-100-insoluble mem- NO is an important paracrine mediator of vascular smooth branes prepared from cultured bovine aortic endothelial cells muscle tone and is an inhibitor of leukocyte adhesion and and colocalizes with caveolin, a coat protein of caveolae, in platelet aggregation (14, 15). -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Dynamin Action at the Final Stage of Clathrin- Mediated Endocytosis
Dynamin Action at the Final Stage of Clathrin- Mediated Endocytosis Ning Fang ( [email protected] ) Georgia State University https://orcid.org/0000-0003-4710-0984 Xiaodong Cheng Georgia State University Kuangcai Chen Georgia State University https://orcid.org/0000-0002-9321-9225 Bin Dong Georgia State University https://orcid.org/0000-0002-3196-0712 Meek Yang Georgia State University Seth Filbrun Georgia State University Yong Myoung Georgia State University Teng-Xiang Huang Georgia State University Yan Gu The Bristol-Myers Squibb Company Gufeng Wang Georgia State University Article Keywords: Dynamin, clathrin-mediated endocytosis, scission, vesicle ssion Posted Date: February 5th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-134570/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/25 Abstract Dynamin plays an important role in clathrin-mediated endocytosis by cutting the neck of nascent vesicles from the cell membrane. Gold nanorods were used as imaging probes to observe dynamin action on cargo vesicles during live endocytosis events. Invariant is that at the peak of dynamin accumulation, the cargo-containing vesicle always gives abrupt, right-handed rotations that nishes in a short time (~ 0.28 s). The large and quick twist, herein named the super twist, is the result of the coordinated dynamin helix action upon GTP hydrolysis. After the super twist, the rotational freedom of the vesicle drastically increases, accompanied with simultaneous or delayed translational movement, indicating that it detaches from the cell membrane. These observations suggest that dynamin-mediated scission at the nal stage involves a large torque generated by coordinated actions of multiple dynamins in the helix, which is the main driving force for scission. -

Membrane Remodeling in Clathrin-Mediated Endocytosis Volker Haucke1,2,* and Michael M
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs216812. doi:10.1242/jcs.216812 REVIEW Membrane remodeling in clathrin-mediated endocytosis Volker Haucke1,2,* and Michael M. Kozlov3,* ABSTRACT membrane fission and vesicle uncoating to eventually allow fusion Clathrin-mediated endocytosis is an essential cellular mechanism by of the nascent endocytic vesicle with endosomes (Box 1). which all eukaryotic cells regulate their plasma membrane composition In spite of important advances in the characterization of these to control processes ranging from cell signaling to adhesion, migration factors, key questions regarding the endocytic process remain. and morphogenesis. The formation of endocytic vesicles and For example, different models have been proposed regarding the tubules involves extensive protein-mediated remodeling of the initiation of clathrin-coated pit (CCP) formation and the onset of plasma membrane that is organized in space and time by protein– membrane curvature acquisition. Moreover, knowledge regarding protein and protein–phospholipid interactions. Recent studies the nanoscale distribution of endocytic proteins within the bilayer combining high-resolution imaging with genetic manipulations of plane during vesicle formation and the mechanisms that guide the endocytic machinery and with theoretical approaches have led their orchestrated assembly and disassembly is only now beginning to novel multifaceted phenomenological data of the temporal and to emerge. Finally, our understanding of the nature and dynamics spatial organization of the endocytic reaction. This gave rise to of membrane phospholipids (Box 2) and their associations with various – often conflicting – models as to how endocytic proteins endocytic proteins during endocytic membrane remodeling and their association with lipids regulate the endocytic protein remains limited. -

Cryo-Tomography of Coat Complexes ISSN 2059-7983
research papers Visualizing membrane trafficking through the electron microscope: cryo-tomography of coat complexes ISSN 2059-7983 Evgenia A. Markova and Giulia Zanetti* Institute of Structural and Molecular Biology, Birkbeck College, Malet Street, London WC1E 7HX, England. Received 8 March 2019 *Correspondence e-mail: [email protected] Accepted 12 April 2019 Coat proteins mediate vesicular transport between intracellular compartments, Keywords: cryo-electron tomography; which is essential for the distribution of molecules within the eukaryotic cell. three-dimensional reconstruction; coat proteins; The global arrangement of coat proteins on the membrane is key to their vesicular transport; COPII; subtomogram function, and cryo-electron tomography and subtomogram averaging have been averaging. used to study membrane-bound coat proteins, providing crucial structural insight. This review outlines a workflow for the structural elucidation of coat proteins, incorporating recent developments in the collection and processing of cryo-electron tomography data. Recent work on coat protein I, coat protein II and retromer performed on in vitro reconstitutions or in situ is summarized. These studies have answered long-standing questions regarding the mechanisms of membrane binding, polymerization and assembly regulation of coat proteins. 1. Introduction Recent advances in cryo-electron microscopy (cryo-EM) have enabled numerous high-resolution studies which have addressed long-standing structural biology questions. The large body of cryo-EM work carried out for protein structure characterization has utilized single-particle electron micro- scopy (Cheng, 2018). Recently, developments in hardware, data collection and data processing have placed cryo-electron tomography (cryo-ET) and subtomogram averaging (STA) at the forefront of structural studies of repetitive assemblies, reaching resolutions comparable to those of single-particle EM (Schur et al., 2016; Wan et al., 2017; Hutchings et al., 2018; Dodonova et al., 2017; Himes & Zhang, 2018). -

Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
www.nature.com/scientificreports OPEN Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Received: 22 October 2018 Accepted: 20 August 2019 Wnt signalling Published: xx xx xxxx Vera Bandmann1, Ann Schirin Mirsanaye1, Johanna Schäfer1, Gerhard Thiel1, Thomas Holstein2 & Melanie Mikosch-Wersching1,2 Receptor-mediated endocytosis is an essential process in signalling pathways for activation of intracellular signalling cascades. One example is the Wnt signalling pathway that seems to depend on endocytosis of the ligand-receptor complex for initiation of Wnt signal transduction. To date, the roles of diferent endocytic pathways in Wnt signalling, molecular players and the kinetics of the process remain unclear. Here, we monitored endocytosis in Wnt3a and Wnt5a-mediated signalling with membrane capacitance recordings of HEK293 cells. Our measurements revealed a swift and substantial increase in the number of endocytic vesicles. Extracellular Wnt ligands specifcally triggered endocytotic activity, which started immediately upon ligand binding and ceased within a period of ten minutes. By using specifc inhibitors, we were able to separate Wnt-induced endocytosis into two independent pathways. We demonstrate that canonical Wnt3a is taken up mainly by clathrin-independent endocytosis whereas noncanonical Wnt5a is exclusively regulated via clathrin-mediated endocytosis. Our fndings show that membrane capacitance recordings allow the resolution of complex cellular processes in plasma membrane signalling pathways in great detail. Wnt signalling is a highly-conserved signalling pathway with important functions in development, tissue-homeostasis, stem cell biology and many diseases, including cancer. Afer three decades of dedicated research we have come to understand many of the fundamental components of Wnt signalling pathways. -
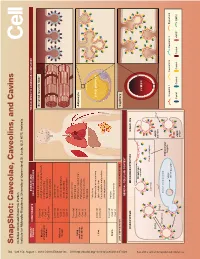
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

A Targeted Rnai Screen Identifies Endocytic Trafficking Factors That
Diabetes Volume 67, March 2018 385 A Targeted RNAi Screen Identifies Endocytic Trafficking Factors That Control GLP-1 Receptor Signaling in Pancreatic b-Cells Teresa Buenaventura,1 Nisha Kanda,1 Phoebe C. Douzenis,1 Ben Jones,2 Stephen R. Bloom,2 Pauline Chabosseau,1 Ivan R. Corrêa Jr.,3 Domenico Bosco,4 Lorenzo Piemonti,5,6 Piero Marchetti,7 Paul R. Johnson,8 A.M. James Shapiro,9 Guy A. Rutter,1 and Alejandra Tomas1 Diabetes 2018;67:385–399 | https://doi.org/10.2337/db17-0639 The glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) is a key binding, GLP-1Rs signal through stimulatory G (Gs) pro- target for type 2 diabetes (T2D) treatment. Because endocytic teins to raise intracellular cAMP levels and modulate insulin trafficking of agonist-bound receptors is one of the most secretion from pancreatic b-cells. important routes for regulation of receptor signaling, a better As with other G protein–coupled receptors (GPCRs), SIGNAL TRANSDUCTION understanding of this process may facilitate the develop- GLP-1R signaling is tightly regulated through endocytic ment of new T2D therapeutic strategies. Here, we screened trafficking (2). Activated GLP-1Rs are rapidly internalized – 29 proteins with known functions in G protein coupled recep- and recycled to the plasma membrane or distributed through- fi tor traf cking for their role in GLP-1R potentiation of insulin out endocytic compartments (3). Despite the pivotal role b fi secretion in pancreatic -cells. We identify ve (clathrin, of trafficking in GPCR signaling, neither the molecular dynamin1, AP2, sorting nexins [SNX] SNX27, and SNX1) details nor the key factors responsible for GLP-1R endo- that increase and four (huntingtin-interacting protein 1 cytosis and postendocytic sorting have been thoroughly [HIP1], HIP14, GASP-1, and Nedd4) that decrease insulin investigated. -

New Perspectives on SNARE Function in the Yeast Minimal Endomembrane System
G C A T T A C G G C A T genes Review New Perspectives on SNARE Function in the Yeast Minimal Endomembrane System James H. Grissom 1, Verónica A. Segarra 2 and Richard J. Chi 1,* 1 Department of Biological Sciences, University of North Carolina at Charlotte, Charlotte, NC 28223, USA; [email protected] 2 Department of Biology, High Point University, High Point, NC 27268, USA; [email protected] * Correspondence: [email protected] Received: 30 June 2020; Accepted: 2 August 2020; Published: 6 August 2020 Abstract: Saccharomyces cerevisiae is one of the best model organisms for the study of endocytic membrane trafficking. While studies in mammalian cells have characterized the temporal and morphological features of the endocytic pathway, studies in budding yeast have led the way in the analysis of the endosomal trafficking machinery components and their functions. Eukaryotic endomembrane systems were thought to be highly conserved from yeast to mammals, with the fusion of plasma membrane-derived vesicles to the early or recycling endosome being a common feature. Upon endosome maturation, cargos are then sorted for reuse or degraded via the endo-lysosomal (endo-vacuolar in yeast) pathway. However, recent studies have shown that budding yeast has a minimal endomembrane system that is fundamentally different from that of mammalian cells, with plasma membrane-derived vesicles fusing directly to a trans-Golgi compartment which acts as an early endosome. Thus, the Golgi, rather than the endosome, acts as the primary acceptor of endocytic vesicles, sorting cargo to pre-vacuolar endosomes for degradation. The field must now integrate these new findings into a broader understanding of the endomembrane system across eukaryotes.