Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

Palmitoylation: Implications for Nitric Oxide Signaling
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6448-6453, June 1996 Cell Biology Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling (endothelial nitric oxide synthase/signal transduction/vascular biology/N-myristoylation) GUILLERMO GARC1A-CARDENA*, PHIL OHt, JIANwEI LIu*, JAN E. SCHNITZERt, AND WILLIAM C. SESSA*t *Molecular Cardiobiology Program and Department of Pharmacology, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536; and tDepartment of Pathology, Harvard Medical School, Beth Israel Hospital, 330 Brookline Avenue, Boston, MA 02215 Communicated by Vincent T. Marchesi, Yale Univeristy, New Haven, CT, March 13, 1996 (received for review February 5, 1996) ABSTRACT The membrane association of endothelial insoluble membranes (TIM), suggesting that caveolae are nitric oxide synthase (eNOS) plays an important role in the signal processing centers (2-11). Additionally, caveolae have biosynthesis of nitric oxide (NO) in vascular endothelium. been implicated in other important cellular functions, includ- Previously, we have shown that in cultured endothelial cells ing endocytosis, potocytosis, and transcytosis (12, 13). and in intact blood vessels, eNOS is found primarily in the Endothelial nitric oxide synthase (eNOS) is a peripheral perinuclear region of the cells and in discrete regions of the membrane protein that metabolizes L-arginine to nitric oxide plasma membrane, suggesting trafficking of the protein from (NO). NO is a short-lived free radical gas involved in diverse the Golgi to specialized plasma membrane structures. Here, physiological and pathological processes. Endothelial-derived we show that eNOS is found in Triton X-100-insoluble mem- NO is an important paracrine mediator of vascular smooth branes prepared from cultured bovine aortic endothelial cells muscle tone and is an inhibitor of leukocyte adhesion and and colocalizes with caveolin, a coat protein of caveolae, in platelet aggregation (14, 15). -

Identification of Caveolin and Caveolin-Related Proteins in the Brain
The Journal of Neuroscience, December 15, 1997, 17(24):9520–9535 Identification of Caveolin and Caveolin-Related Proteins in the Brain Patricia L. Cameron, Johnna W. Ruffin, Roni Bollag, Howard Rasmussen, and Richard S. Cameron Institute of Molecular Medicine and Genetics, Medical College of Georgia, Augusta, Georgia 30912-3175 Caveolae are 50–100 nm, nonclathrin-coated, flask-shaped brane. Immunoblot analyses demonstrate that detergent- plasma membrane microdomains that have been identified in insoluble complexes isolated from astrocytes are composed of most mammalian cell types, except lymphocytes and neurons. caveolin-1a, an identification verified by Northern blot analyses To date, multiple functions have been ascribed to caveolae, and by the cloning of a cDNA using reverse transcriptase-PCR including the compartmentalization of lipid and protein compo- amplification from total astrocyte RNA. Using a full-length nents that function in transmembrane signaling events, biosyn- caveolin-1 probe, Northern blot analyses suggest that the ex- thetic transport functions, endocytosis, potocytosis, and trans- pression of caveolin-1 may be regulated during brain develop- cytosis. Caveolin, a 21–24 kDa integral membrane protein, is ment. Immunoblot analyses of detergent-insoluble complexes the principal structural component of caveolae. We have initi- isolated from cerebral cortex and cerebellum identify two im- ated studies to examine the relationship of detergent-insoluble munoreactive polypeptides with apparent molecular weight and complexes identified -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Internalization of Lectins in Neuronal Gerl
INTERNALIZATION OF LECTINS IN NEURONAL GERL NICHOLAS K. GONATAS, SEUNG U. KIM, ANNA STIEBER, and STRATIS AVRAMEAS From the Division of Neuropathology, Department of Pathology, University of PennsylvaniaSchool of Medicine, Philadelphia, Pennsylvania 19174, and the Laboratory of Immunocytochemistry,Pasteur Institute, Paris, France ABSTRACT Conjugates of ricin agglutinin and phytohemagglutinin with horseradish peroxi- dase (HRP) were used for a cytochemical study of internalization of their plasma membrane "receptors" in cultured isolated mouse dorsal root ganglion neurons. Labeling of cells with lectin-HRP was done at 4~ and internalization was performed at 37~ in a culture medium free of lectin-HRP. 15-30 rain after incubation at 37~ lectin-HRP-receptor complexes were seen in vesicles or tubules located near the plasma membrane. After 1-3 h at 37~ lectin-HRP- receptor complexes accumulated in vesicles and tubules corresponding to acid phosphatase-rich vesicles and tubules (GERL) at the trans aspect of the Golgi apparatus. A few coated vesicles and probably some dense bodies contained HRP after 3-6 h of incubation at 37~ Soluble HRP was not endocytosed under the conditions of this experiment or when it was present in the incubation medium at 37~ Internalization of lectin-HRP-receptor conjugates was decreased or in- hibited by mitochondrial respiration inhibitors but not by cytochalasin B or colchicine. These studies indicate that lectin-labeled plasma membrane moieties of neurons are endocytosed primarily in elements of GERL. Considerable information concerning the mobility tors" for ricin (Ric) and phytohemagglutinin and distribution of plasma membrane proteins has (PHA) labeled with horseradish peroxidase been gained with the use of various ligands. -

Endocytosis of Viruses and Bacteria
Downloaded from http://cshperspectives.cshlp.org/ on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Endocytosis of Viruses and Bacteria Pascale Cossart1 and Ari Helenius2 1Institut Pasteur, Unite´ des Interactions Bacte´ries-Cellules, Paris F-75015, France; INSERM U604, Paris F-75015, France; and INRA, USC2020, Paris F-75015, France 2Institute of Biochemistry, ETH Zurich, 8093 Zurich, Switzerland Correspondence: [email protected]; [email protected] Of the many pathogens that infect humans and animals, a large number use cells of the host organism as protected sites for replication. To reach the relevant intracellular compartments, they take advantage of the endocytosis machinery and exploit the network of endocytic organelles for penetration into the cytosol or as sites of replication. In this review, we discuss the endocytic entry processes used by viruses and bacteria and compare the strategies used by these dissimilar classes of pathogens. any of the most widespread and devastat- valuable insights into fundamental aspects of Ming diseases in humans and livestock are cell biology. caused by viruses and bacteria that enter cells for Here, we focus on the mechanisms by which replication. Being obligate intracellular para- viral and bacterial pathogens exploit the endo- sites, viruses have no choice. They must trans- cytosis machinery for host cell entry and rep- port their genome to the cytosol or nucleus of lication. Among recent reviews on this topic, infected cells to multiply and generate progeny. dedicated uniquely to either mammalian vi- Bacteria and eukaryotic parasites do have other ruses or bacterial pathogens, we recommend options; most of them can replicate on their the following: Cossart and Sansonetti (2004); own. -

Review Caveolae: Where Incoming and Outgoing Messengers Meet Richard G
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10909-10913, December 1993 Review Caveolae: Where incoming and outgoing messengers meet Richard G. W. Anderson Department of Cell Biology and Neuroscience, University of Texas Southwestem Medical Center, Dallas, TX 75235 ABSTIRACT Plasmalemmal caveolae ing. At the same time, this information is This portable, membrane-bound com- were flrst identified as an endocytic com- used to construct several models that partment has been found to contain a partment In endothelial cells, where they illustrate the different ways that caveolae number of molecules that are known to appear to move molecules across the cell might function in both intracellular and participate in cell signaling. There are by transcytosis. More recently, they have intercellular communication. three classes of molecules: enzymes that been found to be sites where small mole- generate messengers from substrates in cules are concentrated and internalized by Caveolae the environment, high-affinity binding a process called potocytosis. A growing sites that concentrate chemical signals, body of biochemical and morphological Each caveola is a dynamic piece ofmem- and substrates that are enzymatically evidence indicates that a variety of mole- brane that is either open for receiving and converted into messengers. cules known to function directly or indi- releasing material or closed for process- GPI. Insulin was the first hormone rectly in signal transduction are enriched ing, storage, and delivery to the cell (11). suspected of using inositol phosphogly- in caveolae. This raises the possibility that The exact nature of the closed compart- can (IPG) or a molecule derived from IPG a third function for caveolae is to process ment is still unclear. -

EFFECT of PHAGOCYTOSIS on MEMBRANE TRANSPORT of NONELECTROLYTES* by MIN-FU TSAN, M.D., and RICHARD D. BERLIN, M.D.:~ (From the D
EFFECT OF PHAGOCYTOSIS ON MEMBRANE TRANSPORT OF NONELECTROLYTES* BY MIN-FU TSAN, M.D., AND RICHARD D. BERLIN, M.D.:~ (From the Department of Physiology, Harvard Medical School, Boston, Massachusetts 02115) (Received for publication 3 June 1971) Phagocytosis and carrier-mediated membrane transport are two distinct membrane functions by which substances are translocated across the plasma membrane. Yet, little is known about the distribution of these functions over the cell surface. During phagocytosis, a part of the plasma membrane is internalized (1, 2). This internalized membrane may include transport sites (carriers). On the other hand, transport sites may be distributed in such a way that different parts of the plasma membrane are involved in transport and phagocytosis. In this study, the activities of specific transport systems were determined before and after large portions of the surface membrane had been interiorized by phagocytosis of inert particles. Five separate transport systems characterized in this laboratory, whose activity can be measured with great sensitivity, have been examined: adenosine 1 and two adenine (3) transport systems in rabbit po]ymorphonuclear leukocytes; adenosine 2 and lysine (4) transport systems in alveolar macrophages. The results show that the rates of transport were un- affected in all systems, even after an estimated 35-50% of the membrane had been internalized. It was also shown that the constancy of transport did not depend on the introduction into the surface of new transport sites during phago- cytosis. The results indicate that the membrane is mosaic in character with geographically separate transport and phagocytic sites. Materials and Methods Animals.--New Zealand white rabbits of either sex, weighing between 2 and 4 kg each, were used. -
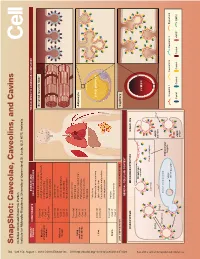
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Exo-Endocytosis at Mossy Fiber Terminals: Toward Capacitance Measurements in Cells with Arbitrary Geometry
Exo-endocytosis at mossy fiber terminals: Toward capacitance measurements in cells with arbitrary geometry Christopher Kushmerick and Henrique von Gersdorff* The Vollum Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239 xocytosis and endocytosis are real time as a decrease in membrane ments have been made on secretory ubiquitous cellular phenomena capacitance back to baseline resting lev- cells for which the compact isopotential necessary for diverse functions els (6–9). approximation seems, prima facie,tobe such as secretion, internal sig- Most measurements obtained to date justified, including adrenal chromaffin Enaling, protein traffic, and motility. have relied on one of two general tech- cells (10), mast cells (11), and neuroen- Many different techniques have been niques to relate membrane current to docrine cells (12), which secrete via developed to assay exocytosis and endo- capacitance (6, 9). Time-domain meth- large dense-core vesicles. In addition, cytosis, but to date only electrical mea- ods use the amplitude and time course small clear-core synaptic vesicle fusion surements of plasma membrane capaci- of membrane current relaxations after and membrane retrieval have been mea- tance have had the time resolution step changes in electrical potential to sured from retinal bipolar cell terminals necessary to capture both the fusion and determine cell membrane parameters. (2, 3, 13), hair cells (4, 5), and photore- reuptake of small clear-core vesicle ceptors (14). However, these sensory membrane during fast neurotransmis- neurons contain nonconventional rib- sion. In this issue of PNAS, Hallermann bon-type active zones (3, 13, 15). et al. (1) present capacitance measure- These are the first Recently, attempts have been made to ments from hippocampal mossy fiber measure exocytosis in cells with complex nerve terminals during stimulated exocy- membrane capacitance geometry and multiple electrical com- tosis. -

Structure and Function of SNARE and SNARE-Interacting Proteins
Quarterly Reviews of Biophysics 38, 1 (2006), pp. 1–47. f 2005 Cambridge University Press 1 doi:10.1017/S0033583505004051 Printed in the United Kingdom First published online 9 December 2005 Structure and functionof SNARE and SNARE-interacting proteins Axel T. Brunger Howard Hughes Medical Institute and Departments of Molecular and Cellular Physiology, Neurology and Neurological Sciences, and Stanford Synchrotron Radiation Laboratory, Stanford University, Stanford, CA 94305, USA Abstract. This review focuses on the so-called SNARE (soluble N-ethyl maleimide sensitive factor attachment protein receptor) proteins that are involved in exocytosis at the pre-synpatic plasma membrane. SNAREs play a role in docking and fusion of synaptic vesicles to the active zone, as well as in the Ca2+-triggering step itself, most likely in combination with the Ca2+ sensor synaptotagmin. Different SNARE domains are involved in different processes, such as regulation, docking, and fusion. SNAREs exhibit multiple configurational, conformational, and oliogomeric states. These different states allow SNAREs to interact with their matching SNARE partners, auxiliary proteins, or with other SNARE domains, often in a mutually exclusive fashion. SNARE core domains undergo progressive disorder to order transitions upon interactions with other proteins, culminating with the fully folded post-fusion (cis) SNARE complex. Physiological concentrations of neuronal SNAREs can juxtapose membranes, and promote fusion in vitro under certain conditions. However, significantly more -

Sodium-Coupled Glucose Transport, the SLC5 Family, and Therapeutically Relevant Inhibitors: from Molecular Discovery to Clinical Application
Pflügers Archiv - European Journal of Physiology (2020) 472:1177–1206 https://doi.org/10.1007/s00424-020-02433-x INVITED REVIEW Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application Gergely Gyimesi1 & Jonai Pujol-Giménez1 & Yoshikatsu Kanai2 & Matthias A. Hediger1 Received: 4 March 2020 /Revised: 24 June 2020 /Accepted: 2 July 2020 / Published online: 7 August 2020 # The Author(s) 2020 Abstract Sodium glucose transporters (SGLTs) belong to the mammalian solute carrier family SLC5. This family includes 12 different members in human that mediate the transport of sugars, vitamins, amino acids, or smaller organic ions such as choline. The SLC5 family belongs to the sodium symporter family (SSS), which encompasses transporters from all kingdoms of life. It furthermore shares similarity to the structural fold of the APC (amino acid-polyamine-organocation) transporter family. Three decades after the first molecular identification of the intestinal Na+-glucose cotransporter SGLT1 by expression cloning, many new discoveries have evolved, from mechanistic analysis to molecular genetics, structural biology, drug discovery, and clinical applications. All of these advances have greatly influenced physiology and medicine. While SGLT1 is essential for fast absorption of glucose and galactose in the intestine, the expression of SGLT2 is largely confined to the early part of the kidney proximal tubules, where it reabsorbs the bulk part of filtered glucose. SGLT2 has been successfully exploited by the pharmaceutical industry to develop effective new drugs for the treatment of diabetic patients. These SGLT2 inhibitors, termed gliflozins, also exhibit favorable nephroprotective effects and likely also cardioprotective effects.