Palmitoylation: Implications for Nitric Oxide Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

Identification of Caveolin and Caveolin-Related Proteins in the Brain
The Journal of Neuroscience, December 15, 1997, 17(24):9520–9535 Identification of Caveolin and Caveolin-Related Proteins in the Brain Patricia L. Cameron, Johnna W. Ruffin, Roni Bollag, Howard Rasmussen, and Richard S. Cameron Institute of Molecular Medicine and Genetics, Medical College of Georgia, Augusta, Georgia 30912-3175 Caveolae are 50–100 nm, nonclathrin-coated, flask-shaped brane. Immunoblot analyses demonstrate that detergent- plasma membrane microdomains that have been identified in insoluble complexes isolated from astrocytes are composed of most mammalian cell types, except lymphocytes and neurons. caveolin-1a, an identification verified by Northern blot analyses To date, multiple functions have been ascribed to caveolae, and by the cloning of a cDNA using reverse transcriptase-PCR including the compartmentalization of lipid and protein compo- amplification from total astrocyte RNA. Using a full-length nents that function in transmembrane signaling events, biosyn- caveolin-1 probe, Northern blot analyses suggest that the ex- thetic transport functions, endocytosis, potocytosis, and trans- pression of caveolin-1 may be regulated during brain develop- cytosis. Caveolin, a 21–24 kDa integral membrane protein, is ment. Immunoblot analyses of detergent-insoluble complexes the principal structural component of caveolae. We have initi- isolated from cerebral cortex and cerebellum identify two im- ated studies to examine the relationship of detergent-insoluble munoreactive polypeptides with apparent molecular weight and complexes identified -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Folate Receptors Targeted to Clathrin-Coated Pits Cannot Regulate Vitamin Uptake
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 3824-3828, April 1995 Cell Biology Folate receptors targeted to clathrin-coated pits cannot regulate vitamin uptake (caveolae/glycosyl-phosphatidylinositol/potocytosis/endocytosis/5-methyltetrahydrofolate) TIMOTHY E. RITTER*, OSVALDo FAJARDO*, HIROYUKI MATSUEt, RICHARD G. W. ANDERSONt, AND STEPHEN W. LACEY*§ Departments of *Internal Medicine, tDermatology and Cell Biology, and tNeuroscience, University of Texas Southwestern Medical Center, Dallas, TX 75235 Communicated by Michael S. Brown, University of Texas Southwestern Medical Center, Dallas, TX, January 3, 1995 ABSTRACT Potocytosis is an endocytic process that is coated pits (6). Clearly it is not the initial internalization step specialized for the internalization of small molecules. Recent that is advantageous, because the rate of ligand sequestration studies on the uptake of5-methyltetrahydrofolate by the folate by caveolae is about 5 times slower (17) than that by clathrin- receptor have suggested that the glycosyl-phosphatidylinositol coated pits (18). Nothing is known, however, about how the anchor on this protein causes it to cluster and be internalized two pathways might differ in either the efficiency of ligand by caveolae instead of coated pits. To test this hypothesis delivery to the cytoplasm or the regulation of folate accumu- directly, we have constructed a chimeric folate receptor that lation in the cytoplasm. We have addressed these two questions has the glycosyl-phosphatidylinositol anchor replaced with by constructing a chimeric folate receptor that is internalized the transmembrane domain and cytoplasmic tail of the low by clathrin-coated pits and the comparing uptake of 5-MeTHF density lipoprotein receptor. The cells with wild-type recep- by this receptor with uptake by the wild-type receptor in tors delivered 5-methyltetrahydrofolate to the cytoplasm more transfected cells. -

Review Caveolae: Where Incoming and Outgoing Messengers Meet Richard G
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10909-10913, December 1993 Review Caveolae: Where incoming and outgoing messengers meet Richard G. W. Anderson Department of Cell Biology and Neuroscience, University of Texas Southwestem Medical Center, Dallas, TX 75235 ABSTIRACT Plasmalemmal caveolae ing. At the same time, this information is This portable, membrane-bound com- were flrst identified as an endocytic com- used to construct several models that partment has been found to contain a partment In endothelial cells, where they illustrate the different ways that caveolae number of molecules that are known to appear to move molecules across the cell might function in both intracellular and participate in cell signaling. There are by transcytosis. More recently, they have intercellular communication. three classes of molecules: enzymes that been found to be sites where small mole- generate messengers from substrates in cules are concentrated and internalized by Caveolae the environment, high-affinity binding a process called potocytosis. A growing sites that concentrate chemical signals, body of biochemical and morphological Each caveola is a dynamic piece ofmem- and substrates that are enzymatically evidence indicates that a variety of mole- brane that is either open for receiving and converted into messengers. cules known to function directly or indi- releasing material or closed for process- GPI. Insulin was the first hormone rectly in signal transduction are enriched ing, storage, and delivery to the cell (11). suspected of using inositol phosphogly- in caveolae. This raises the possibility that The exact nature of the closed compart- can (IPG) or a molecule derived from IPG a third function for caveolae is to process ment is still unclear. -

Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
www.nature.com/scientificreports OPEN Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Received: 22 October 2018 Accepted: 20 August 2019 Wnt signalling Published: xx xx xxxx Vera Bandmann1, Ann Schirin Mirsanaye1, Johanna Schäfer1, Gerhard Thiel1, Thomas Holstein2 & Melanie Mikosch-Wersching1,2 Receptor-mediated endocytosis is an essential process in signalling pathways for activation of intracellular signalling cascades. One example is the Wnt signalling pathway that seems to depend on endocytosis of the ligand-receptor complex for initiation of Wnt signal transduction. To date, the roles of diferent endocytic pathways in Wnt signalling, molecular players and the kinetics of the process remain unclear. Here, we monitored endocytosis in Wnt3a and Wnt5a-mediated signalling with membrane capacitance recordings of HEK293 cells. Our measurements revealed a swift and substantial increase in the number of endocytic vesicles. Extracellular Wnt ligands specifcally triggered endocytotic activity, which started immediately upon ligand binding and ceased within a period of ten minutes. By using specifc inhibitors, we were able to separate Wnt-induced endocytosis into two independent pathways. We demonstrate that canonical Wnt3a is taken up mainly by clathrin-independent endocytosis whereas noncanonical Wnt5a is exclusively regulated via clathrin-mediated endocytosis. Our fndings show that membrane capacitance recordings allow the resolution of complex cellular processes in plasma membrane signalling pathways in great detail. Wnt signalling is a highly-conserved signalling pathway with important functions in development, tissue-homeostasis, stem cell biology and many diseases, including cancer. Afer three decades of dedicated research we have come to understand many of the fundamental components of Wnt signalling pathways. -
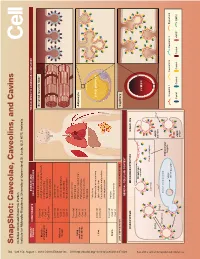
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Exocytosis and Endocytosis
Exocytosis and Endocytosis Exocytosis and Endocytosis A Closer Look at Cell Membranes . Aim: How do large particles enter and exit cells? . Do Now: Name some molecules/materials that enter and exit the cell. How would you describe the cell membrane that allows passage of these materials? Exocytosis and Endocytosis Exocytosis and Endocytosis . Exocytosis (out of the cell) • The fusion of a vesicle with the cell membrane, releasing its contents to the surroundings . Endocytosis (into the cell) • The formation of a vesicle from cell membrane, enclosing materials near the cell surface and bringing them into the cell Exocytosis and Endocytosis Endocytosis . Phagocytosis – solid . Pinocytosis – liquid (general) Endocytosis: . Uptake of substances . Transport of protein or lipid components of compartments . Metabolic or division signaling . Defense to microorganisms Endocytosis . Clathrin-coated vesicles . Non-clathrin coated vesicles . Macropinocytosis . Potocytosis Exocytosis and Endocytosis Endocytosis Required: . signal . membrane receptor (Fc receptor for Ab) . formation of pseudopodium . cortical actin network The formed vesicle: phagosome (hetero-; auto-) Endocytosis . Clathrin-coated vesicles . Non-clathrin coated vesicles . Macropinocytosis . Potocytosis Endocytosis and Exocytosis Examples Three Pathways of Endocytosis . Bulk-phase endocytosis • Extracellular fluid is captured in a vesicle and brought into the cell; the reverse of exocytosis . Receptor-mediated endocytosis • Specific molecules bind to surface receptors, which are then enclosed in an endocytic vesicle . Phagocytosis • Pseudopods engulf target particle and merge as a vesicle, which fuses with a lysosome in the cell Phagocytosis (“engulfment”) Exocytosis and Endocytosis Membrane Cycling . Exocytosis and endocytosis continually replace and withdraw patches of the plasma membrane . New membrane proteins and lipids are made in the ER, modified in Golgi bodies, and form vesicles that fuse with plasma membrane Exocytic Vesicle 5.5 Key Concepts: Membrane Trafficking . -

Structure and Function of SNARE and SNARE-Interacting Proteins
Quarterly Reviews of Biophysics 38, 1 (2006), pp. 1–47. f 2005 Cambridge University Press 1 doi:10.1017/S0033583505004051 Printed in the United Kingdom First published online 9 December 2005 Structure and functionof SNARE and SNARE-interacting proteins Axel T. Brunger Howard Hughes Medical Institute and Departments of Molecular and Cellular Physiology, Neurology and Neurological Sciences, and Stanford Synchrotron Radiation Laboratory, Stanford University, Stanford, CA 94305, USA Abstract. This review focuses on the so-called SNARE (soluble N-ethyl maleimide sensitive factor attachment protein receptor) proteins that are involved in exocytosis at the pre-synpatic plasma membrane. SNAREs play a role in docking and fusion of synaptic vesicles to the active zone, as well as in the Ca2+-triggering step itself, most likely in combination with the Ca2+ sensor synaptotagmin. Different SNARE domains are involved in different processes, such as regulation, docking, and fusion. SNAREs exhibit multiple configurational, conformational, and oliogomeric states. These different states allow SNAREs to interact with their matching SNARE partners, auxiliary proteins, or with other SNARE domains, often in a mutually exclusive fashion. SNARE core domains undergo progressive disorder to order transitions upon interactions with other proteins, culminating with the fully folded post-fusion (cis) SNARE complex. Physiological concentrations of neuronal SNAREs can juxtapose membranes, and promote fusion in vitro under certain conditions. However, significantly more -
Concentration Gradient; Within a System, Different Substances in the Medium Will Each Diffuse at Different Rates According to Their Individual Gradients
Biomolecules Biological Macromolecules • Life depends on four types of organic macromolecules: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids 1. Carbohydrates • Contain carbon, hydrogen and oxygen in a ratio of 1:2:1 • Account for less that 1% of body weight • Used as energy source • Called saccharides Carbohydrates • Compounds containing C, H and O • General formula : Cx(H2O)y • All have C=O and -OH functional groups. • Classified based on • Size of base carbon chain • Number of sugarunits • Location of C=O • Stereochemistry Types of carbohydrates • Classifications based on number of sugarunits in total chain. • Monosaccharides - single sugarunit • Disaccharides - two sugarunits • Oligosaccharides - 2 to 10 sugarunits • Polysaccharides - more than 10units • Chaining relies on ‘bridging’ of oxygenatoms • glycoside bonds Monosaccharides • Based on location of C=O H CH2OH | | C=O C=O | | H-C-OH HO-C-H | | H-C-OH H-C-OH | | H-C-OH H-C-OH | | CH2OH CH2OH Aldose Ketose - aldehyde C=O - ketone C=O Monosaccharide classifications • Number of carbon atoms in the chain H H | H | C=O H | C=O | | C=O | H-C-OH C=O | H-C-OH | | H-C-OH | H-C-OH | H-C-OH | H-C-OH H-C-OH | H-C-OH | | H-C-OH | CH2OH | H-C-OH CH2OH | CH2OH CH2OH triose tetrose pentose hexose Can be either aldose or ketose sugar. Stereoisomers • Stereochemistry • Study of the spatial arrangement ofmolecules. • Stereoisomers have • the same order and types of bonds. • different spatial arrangements. • different properties. • Many biologically importantchemicals, like sugars, exist as stereoisomers. Your body can tell the difference. -
Modulation of the Caveolin-3 Localization to Caveolae and STAT3 to Mitochondria by Catecholamine-Induced Cardiac Hypertrophy in H9c2 Cardiomyoblasts
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 41, No. 4, 226-235, April 2009 Modulation of the caveolin-3 localization to caveolae and STAT3 to mitochondria by catecholamine-induced cardiac hypertrophy in H9c2 cardiomyoblasts Kyuho Jeong*, Hayeong Kwon*, amine-induced cardiac hypertrophy. Chanhee Min and Yunbae Pak1 Keywords: cardiomegaly; caveolae; caveolin-3; cell Department of Biochemistry nucleus; heart; isoproterenol; mitochondria; phenyl- Division of Applied Life Science (BK21), PMBBRC ephrine; STAT3 transcription factor Environmental Biotechnology National Core Research Center Gyeongsang National University Jinju 660-701, Korea Introduction 1Corresponding author: Tel, 82-55-751-5961; Fax, 82-55-759-9363; E-mail, [email protected] Hypertension is major risk factors for cardiac da- mage, ischemia, myocardial infarction, and conge- *These authors contributed equally to this work. stive heart failure (Zampaglione et al., 1996). In DOI 10.3858/emm.2009.41.4.025 response to increased demands for cardiac work caused by various pathologic stresses, heart adapts Accepted 20 November 2008 through compensatory hypertrophy of myocytes. Abbreviations: Akt, protein kinase B; CsA, cyclosporin A; GPCR, Thus, cardiac hypertrophy is recognized in many G protein-coupled receptor; ISO, isoproterenol; PE, phenylephrine; cardiovascular diseases, such as hypertension, RTK, receptor tyrosine kinase; STAT3, signal transducers and acti- vascular disease, and myocardial infarction, and is vator of transcription 3 an independent risk factor for cardiac morbidity and mortality. Hypertrophic stimuli induce an in- crease in cell size in the absence of cell division through Ca2+ signaling and activation of PKC, Abstract MAPK and PKB/ Akt (Watanabe et al., 2001; Dorn and Force, 2005), and are accompanied by We investigated the effect of phenylephrine (PE)- and increased protein synthesis with reprogramming of isoproterenol (ISO)-induced cardiac hypertrophy on gene expression (Takeo et al., 2000). -

Three Ways to Make a Vesicle
REVIEWS THREE WAYS TO MAKE A VESICLE Tomas Kirchhausen Cargo molecules have to be included in carrier vesicles of different forms and sizes to be transported between organelles. During this process, a limited set of proteins, including the coat proteins COPI, COPII and clathrin, carries out a programmed set of sequential interactions that lead to the budding of vesicles. A general model to explain the formation of coated vesicles is starting to emerge but the picture is more complex than we had imagined. ENDOCYTIC PATHWAY Transport of proteins and lipids along the ENDOCYTIC or energy dependent and includes sorting of cargo to the Macromolecules are taken up SECRETORY PATHWAYS is a hallmark of eukaryotic cells. The forming coat. Coat propagation, the second step in the by invagination of the plasma membrane fluxes along these pathways are very large process, couples further addition of coat components membrane. They first arrive in and rapid. A FIBROBLAST kept in resting conditions in a and additional recruitment of cargo with invagination of early endosomes, then late endosomes, and finally tissue culture plate internalizes an amount of mem- the underlying membrane. When formation of the coat lysosomes, where they are brane equivalent to the whole surface area of the cell in ends, the vesicle buds by scission of the neck connecting degraded by hydrolases. one hour. Inside the cell, it often takes only seconds for a the deeply invaginated membrane to the donor surface. carrier vesicle to move from the donor membrane to This is a relatively simple step for COPI- and COPII- SECRETORY PATHWAY the acceptor organelle.